Drug delivery
Micro / nanoparticles
Controlled release of protein from degradable multi-block copolymer microspheres.
About the project:
Contact:
Collaborations:
Pr Venier and Dr Montero-Venei (Micro et Nanomédecines Translationnelles , MINT UMR 1066-CNRS 6021)
Funding:
None
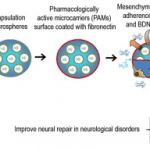
Pharmacologically active microcarriers delivering BDNF within a hydrogel: Novel strategy for human bone marrow-derived stem cells neural neuronal differentiation guidance and therapeutic secretome enhancement
This is custom heading element
Acta Biomater. 49, 167–180 (2017)
Kandalam, S., Sindji, L., Delcroix, G. J.-R., Violet, F., Garric, X., Andre, E. M., Schiller, P. C., Venier-Julienne, M.-C., des Rieux, A., Guicheux, J. & Montero-Menei, C. N.
ABSTRACT
Stem cells combined with biodegradable injectable scaffolds releasing growth factors hold great promises in regenerative medicine, particularly in the treatment of neurological disorders. We here integrated human marrow-isolated adult multilineage-inducible (MIAMI) stem cells and pharmacologically active microcarriers (PAMs) into an injectable non-toxic silanized-hydroxypropyl methylcellulose (Si-HPMC) hydrogel. The goal is to obtain an injectable non-toxic cell and growth factor delivery device. It should direct the survival and/or neuronal differentiation of the grafted cells, to safely transplant them in the central nervous system, and enhance their tissue repair properties. A model protein was used to optimize the nanoprecipitation conditions of the neuroprotective brain-derived neurotrophic factor (BDNF). BDNF nanoprecipitate was encapsulated in fibronectin-coated (FN) PAMs and the in vitro release profile evaluated. It showed a prolonged, bi-phasic, release of bioactive BDNF, without burst effect. We demonstrated that PAMs and the Si-HPMC hydrogel increased the expression of neural/neuronal differentiation markers of MIAMI cells after 1 week. Moreover, the 3D environment (PAMs or hydrogel) increased MIAMI cells secretion of growth factors (b-NGF, SCF, HGF, LIF, P1GF-1, SDF-1 alpha, VEGF-A & D) and chemokines (MIP-la & RANTES, IL-8). These results show that PAMs delivering BDNF combined with Si-HPMC hydrogel represent a useful novel local delivery tool in the context of neurological disorders. It not only provides neuroprotective BDNF but also bone marrow-derived stem cells that benefit from that environment by displaying neural commitment and an improved neuroprotective/reparative secretome. It provides preliminary evidence of a promising pro-angiogenic, neuroprotective and axonal growth-promoting device for the nervous system. Statement of Significance Combinatorial tissue engineering strategies for the central nervous system are scarce. We developed and characterized a novel injectable non-toxic stem cell and protein delivery system providing regenerative cues for central nervous system disorders. BDNF, a neurotrophic factor with a wide-range effect, was nanoprecipitated to maintain its structure and released in a sustained manner from novel polymeric microcarriers. The combinatorial 3D support, provided by fibronectin-microcarriers and the hydrogel, to the mesenchymal stem cells guided the cells towards a neuronal differentiation and enhanced their tissue repair properties by promoting growth factors and cytokine secretion. The long-term release of physiological doses of bioactive BDNF, combined to the enhanced secretion of tissue repair factors from the stem cells, constitute a promising therapeutic approach.
Congratulations to Jean Coudane for the GFP 2024 honorary award
This is custom heading element

Congratulation Jean!
Your investment in the community and your scientific contribution were rewarded by the GFP 2024, which awarded you the honorary prize!
Congratulations to Anissa Benkhedim for winning the Best student Poster Award
This is custom heading element

Congratulation Anissa!
Your work and the presentation of your poster were rewarded by the “Polymers for a safe and sustainable Future” conference.
Congratulations for your prize : best poster award!
Congratulations to Hélène Van den Berghe for obtaining the HDR
This is custom heading element

We are proud to celebrate an important moment in the life of our team. Today, we would like to extend our warmest congratulations to Hélène Van den Berghe, assistant professor at University of Montpellier on obtaining the HDR. We wish you the best for the rest of your carrier.
Bravo!
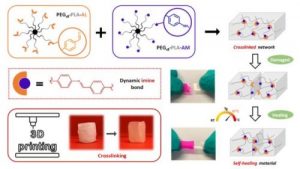
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet
ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Novel Biopolymers for Sustained Delivery of Drugs to the Eye
Micelles:
Multifunctional polyesters for stimuli-responsive drug delivery systems
About the project:
This project is dedicated to the design and synthesis of multifunctional and degradable amphiphilic copolymers for drug delivery applications. Original architectures are in particular investigated and compared to classical linear copolymers.

Contact:
Students:


Collaborations:
Prof. Bakkour (Lebanese University, Lebanon) Dr. Etrych, Dr. Koziolova, Dr. Janouskova (Institute of Macromolecular Chemistry, Czech Republic), Dr. Coll (University Grenoble-Alpes), Prof. Subra (IBMM-peptides, UMR 5247), Dr. Pound-Lana & Prof. Mosqueira (Federal University of Ouro Preto, Brazil)
Funding:
Doctoral grants for LASER and Azm & Saade Associations
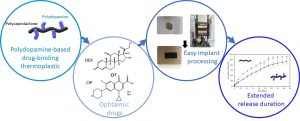
Polyester-polydopamine copolymers for intravitreal drug delivery: role of polydopamine drug-binding properties on extending drug release
This is custom heading element
Biomacromolecules 23, 4388-4400, (2022)
Floriane Bahuon, Vincent Darcos, Sulabh Patel, Zana Marin, Jean Coudane, Grégoire Schwach, and Benjamin Nottelet
ABSTRACT
This work reports on a novel polyester copolymer containing poly(dopamine), a synthetic analogue of natural melanin, evaluated in sustained-release drug delivery system for ocular intravitreal administration of drugs. More specifically, a graft copolymer of poly(ε-caprolactone)-graft-poly(dopamine) (PCL-g-PDA) has been synthesized, and was shown to further extend the drug release benefits of state-of-the-art biodegradable intravitreal implants made of poly(lactide) and poly(lactide-co-glycolide). The innovative biomaterial combines the documented drug-binding properties of melanin naturally present in the eye, with the established ocular tolerability and biodegradation of polyester implants. The PCL-g-PDA copolymer was obtained by a two-step modification of PCL with a final PDA content around 2-3 wt.%, and was fully characterised by SEC, NMR, and DOSY NMR. The thermoplastic nature of PCL-g-PDA allowed its simple processing by hot-melt compression moulding to prepare small implants. The properties of unmodified PCL and PCL-g-PDA implants were studied and compared in terms of thermal properties (DSC), thermal stability (TGA), degradability and in vitro cytotoxicity. PCL and PCL-g-PDA implants exhibited similar degradation properties in vitro and were both stable under physiological conditions over 110 days. Likewise, both materials were non-cytotoxic towards L929 and ARPE-19 cells. The drug-loading and in vitro release properties of the new materials were investigated with dexamethasone (DEX) and ciprofloxacin hydrochloride (CIP) as representative drugs featuring low and high melanin binding affinities, respectively. In comparison to unmodified PCL, PCL-g-PDA implants showed significant extension of drug release most likely because of specific drug-catechol interaction with the PDA moieties of the copolymer. The present study confirms the advantages of designing PDA-containing polyesters as a class of biodegradable and biocompatible thermoplastics that can modulate and remarkably extend drug release kinetics thanks to their unique drug binding properties, especially, but not limited to, for ocular applications.
Peptide-guided self-assembly of polyethylene glycol-b-poly(ε-caprolactone-g-peptide) block copolymers
This is custom heading element
Eur. Pol. J. 176, 111386 (2022)
Ayman El Jundi, Matthias Mayor, Enrique Folgado, Chaimaa Gomri, Belkacem Tarek Benkhaled, Arnaud Chaix, Pascal Verdie, Benjamin Nottelet, Mona Semsarilar

ABSTRACT
Biodegradable poly(ethylene glycol)-b-poly(ε-caprolactone-g-peptide) (PEG-b-PCL-g-peptide) copolymers were synthesized using a combination of ring opening polymerization and thiol-yne photoaddition of peptides on the alkyne functional PCL block. The peptides Phe-Phe, Tyr-Tyr and Arg-Gly-Asp were selected based on the expected interactions (Pi-stacking, H-bonding, electrostatic). The self-assembly of these copolymers was studied via testing the effect of various parameters such as the nature of the solvent and non-solvant as well as their ratio,mixing method, temperature and concentration. Structures obtained by varying these parameters were characterised using transmission electron microscopy (TEM) and dynamic light scattering (DLS). Spherical and lamellar structures (oval leaf-shaped) of different sizes were identified as a function of the conditions. The role of the crystallisation and of the peptides was highlighted with more defined and stable structures obtained for Tyr-Tyr functional copolymers.
Double hydrophilic block copolymers self-assemblies in biomedical applications
This is custom heading element
Adv. Colloid Interface Sci 283, 102213, (2020)
A. El Jundi, S. Buwalda, Y. Bakkour, X. Garric, B. Nottelet
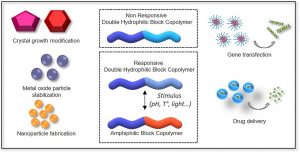
ABSTRACT
Double-hydrophilic block copolymers (DHBCs), consisting of at least two different water-soluble blocks, are an alternative to the classical amphiphilic block copolymers and have gained increasing attention in the field of biomedical applications. Although the chemical nature of the two blocks can be diverse, most classical DHBCs consist of a bioeliminable non-ionic block to promote solubilization in water, like poly(ethylene glycol), and a second block that is more generally a pH-responsive block capable of interacting with another ionic polymer or substrate. This second block is generally non-degradable and the presence of side chain functional groups raises the question of its fate and toxicity, which is a limitation in the frame of biomedical applications. In this review, following a first part dedicated to recent examples of non-degradable DHBCs, we focus on the DHBCs that combine a biocompatible and bioeliminable non-ionic block with a degradable functional block including polysaccharides, polypeptides, polyesters and other miscellaneous polymers. Their use to design efficient drug delivery systems for various biomedical applications through stimuli-dependent self-assembly is discussed along with the current challenges and future perspectives for this class of copolymers.
Degradable double hydrophilic block copolymers and tripartite polyionic complex micelles thereof for small interfering ribonucleic acids (siRNA) delivery
This is custom heading element
J. Colloid Interface 580, 449, (2020)
A. El Jundi, M. Morille, N. Bettache, A. Bethry, J. Berthelot, J. Salvador, S. Hunger, Y. Bakkour, E. Belamie, B. Nottelet
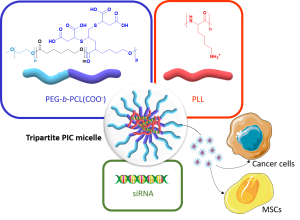
ABSTRACT
Polymer vectors for gene therapy have been largely investigated as an alternative to viral vectors. In particular, double hydrophilic block copolymers (DHBCs) have shown potential in this domain, but to date studies mainly focus on non-degradable copolymers, which may be a restriction for further development. To overcome this limitation, we synthesized a DHBC (PEG43-b-PCL12(COOH)6.5) composed of a poly(ethylene glycol) (PEG) non-ionic and bioeliminable block and a degradable carboxylic acid-functionalized poly(e-caprolactone) (PCL) block. The potential of this DHBC as an original vector for small interfering ribonucleic acids (siRNA) to formulate tripartite polyionic complex (PIC) micelles with poly(lysine) (PLL) was evaluated. We first studied the impact of the charge ratio (R) on the size and the zeta potential of the resulting micelles. With a charge ratio R=1, one formulation with optimized physico-chemical properties showed the ability to complex 75 % of siRNA. We showed a stability of the micelles at pH 7.4 and a disruption at pH 5, which allowed a pH-triggered siRNA release and proved the pH-stimuli responsive character of the tripartite micelles. In addition, the tripartite PIC micelles were shown to be non-cytotoxic below 40 µg/mL. The potential of these siRNA vectors was further evaluated in vitro: it was found that the tripartite PIC micelles allowed siRNA internalization to be 3 times higher than PLL polyplexes in murine mesenchymal stem cells, and were able to transfect human breast cancer cells. Overall, this set of data pre-validates the use of degradable DHBC as non-viral vectors for the encapsulation and the controlled release of siRNA, which may therefore constitute a sound alternative to non-degradable and/or cytotoxic polycationic vectors.
Stabilized micelles for prolonged drug delivery
About the project:
In this project, we take advantage of weak interactions (Pi-Pi stacking, hydrogen bonding, coordination) to stabilize micellar systems in order to increase their therapeutic benefice.
Contact:
Students:



Collaborations:
Dr. Kok (Utrecht University)
Funding:
Marie Skłodowska-Curie grant, doctoral grants for LASER and Azm & Saade Associations
Reversibly core-crosslinked PEG-P(HPMA) micelles: Platinum coordination chemistry for competitive-ligand-regulated drug delivery
This is custom heading element
J. Colloid Interface Sci. 535, 505–515 (2019)
Buwalda, S., Nottelet, B., Bethry, A., Kok, R. J., Sijbrandi, N. & Coudane, J.
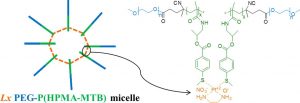
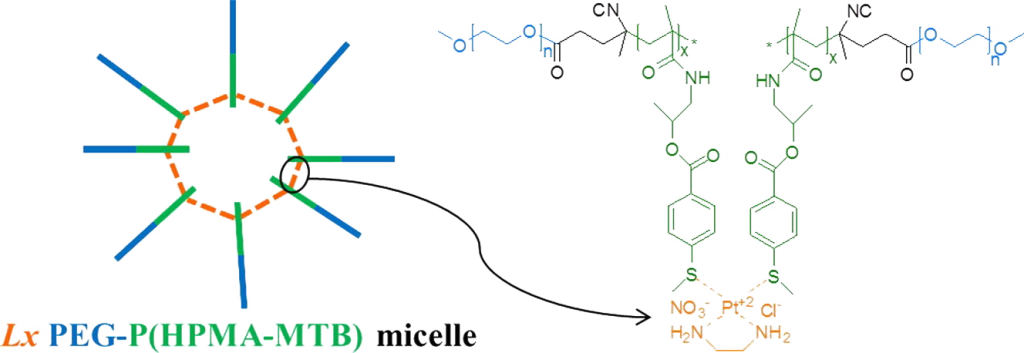
ABSTRACT
Hypothesis. The presence of pendant thioether groups on poly(ethylene glycol)-poly(N(2-hydroxypropyl) methacrylamide) (PEG-P(HPMA)) block copolymers allows for platinum-mediated coordinative micellar core-crosslinking, resulting in enhanced micellar stability and stimulus-responsive drug delivery.
Experiments. A new PEG-P(HPMA) based block copolymer with pendant 4-(methylthio)benzoyl (MTB) groups along the P(HPMA) block was synthesized by free radical polymerization of a novel HPMA-MTB monomer using a PEG based macro-initiator. As crosslinker the metal-organic linker [ethylenediamineplatinum(II)]2+ was used, herein called Lx, which is a coordinative linker molecule that has been used for the conjugation of drug molecules to a number of synthetic or natural carrier systems such as hyperbranched polymers and antibodies.
Findings. The introduction of Lx in the micellar core results in a smaller size, a lower critical micelle concentration and a better retention of the hydrophobic drug curcumin thanks to coordination bonds between the central platinum atom of Lx and thioether groups on different polymer chains. The drug release from Lx crosslinked micelles is significantly accelerated under conditions mimicking the intracellular environment due to competitive coordination and subsequent micellar de-crosslinking. Because of their straightforward preparation and favorable drug release characteristics, core-crosslinked Lx PEG-P(HPMA) micelles hold promise as a versatile nanomedicine platform.
Stabilization of poly(ethylene glycol)-poly(epsilon-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties
This is custom heading element
Colloid Interface Sci. 514, 468–478 (2018)
Buwalda, S., Al Samad, A., El Jundi, A., Bethry, A., Bakkour, Y., Coudane, J. & Nottelet, B
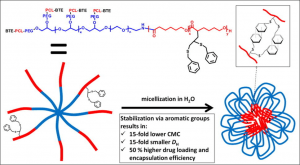
ABSTRACT
Hypothesis
The functionalization of poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) block copolymers with moieties allowing for core-crosslinking is expected to result in improved micellar stability and drug delivery properties.
Experiments
PEG-(PCL)8 star block copolymers were functionalized with pendant benzylthioether (BTE) groups by applying an anionic post-polymerization modification technique followed by photoradical thiol-yne addition of benzyl mercaptan. The micellar properties of PEG-(PCL)8 and PEG-(PCL-BTE)8 were studied and compared in terms of critical micelle concentration (CMC), size, morphology, drug loading and release and in vitro cytotoxicity.
Findings
In comparison with unmodified PEG-(PCL)8 micelles, PEG-(PCL-BTE)8 micelles exhibited a 15-fold lower CMC, a 15-fold smaller size and a 50% higher drug loading and encapsulation efficiency thanks to the presence of pendant benzyl groups which provide the possibility for micellar core-crosslinking via supramolecular π-π stacking and additional hydrophobic interactions. Whereas the PEG-(PCL)8 micelles showed significant aggregation during in vitro cytotoxicity experiments, the PEG-(PCL-BTE)8 micelles showed no signs of aggregation and were capable of solubilizing high concentrations of curcumin, resulting in a significant decrease in MCF-7 cell viability after 48 h. Their ease of synthesis combined with promising results regarding drug delivery make the PEG-(PCL-BTE)8 micelles appealing for application in the field of encapsulation.
Polyester-g-oligosaccharide micelles for cancer therapy
About the project:
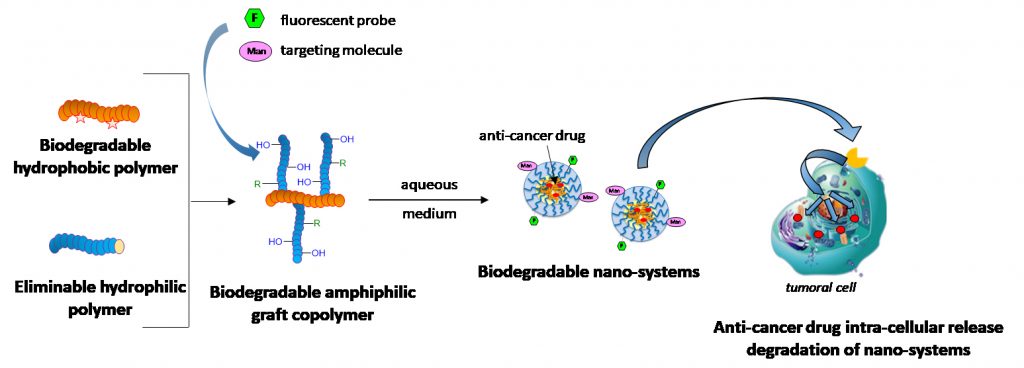
Contact:
Students:

Collaborations:
Dr. Magali Gary-Bobo, Pr Alain Morère Kok (« Glyco and nanovectors for therapeutic targeting » team, IBMM, University of Montpellier), Dr. Ghislain David (IAM team, ICGM, University of Montpellier)
Funding:
“Emergence project” funding from the “Cancéropôle Grand Sud Ouest”, Doctoral Grant from the French Ministry
Congratulations to Jean Coudane for the GFP 2024 honorary award
This is custom heading element

Congratulation Jean!
Your investment in the community and your scientific contribution were rewarded by the GFP 2024, which awarded you the honorary prize!
Congratulations to Anissa Benkhedim for winning the Best student Poster Award
This is custom heading element

Congratulation Anissa!
Your work and the presentation of your poster were rewarded by the “Polymers for a safe and sustainable Future” conference.
Congratulations for your prize : best poster award!
Congratulations to Hélène Van den Berghe for obtaining the HDR
This is custom heading element

We are proud to celebrate an important moment in the life of our team. Today, we would like to extend our warmest congratulations to Hélène Van den Berghe, assistant professor at University of Montpellier on obtaining the HDR. We wish you the best for the rest of your carrier.
Bravo!
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Thermo-responsive poly(lactic acid)-base micelles for drug delivery
About the project:
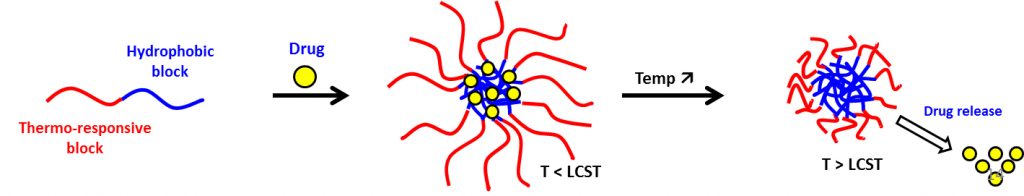
Contact:
Students:

Collaborations:
Prof. Sébastien Lecommendoux (LCPO, Bordeaux)
Funding:
CNRS
Thermo-responsive drug release from self-assembled micelles of brush-like PLA/PEG analogues block copolymers
This is custom heading element
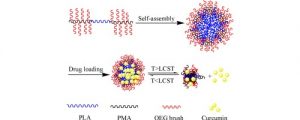
ABSTRACT
Thermo-responsive brush-like amphiphilic poly[2-(2-methoxyethoxy) ethyl methacrylate-co-oligo(ethylene glycol) methacrylate]-b-poly(l-lactide)-b-poly[2-(2-methoxyethoxy) ethyl methacrylate-co-oligo(ethylene glycol) methacrylate] [P(MEO2MA-co-OEGMA)-b-PLLA-b-P(MEO2MA-co-OEGMA)] triblock copolymers were synthesized by atom transfer radical polymerization of MEO2MA and OEGMA co-monomers using a α,ω-Bromopropionyl poly(l-lactide) (Br-PLLA-Br) macroinitiator. The resulting copolymers with MEO2MA/OEGMA molar ratio ranging from 79/21 to 42/58 were characterized by 1H nuclear magnetic resonance and size exclusion chromatography. Thermo-responsive micelles were obtained by self-assembly of copolymers in aqueous medium. The micelles are spherical in shape with sizes varying from 20.7 to 102.5 nm. A hydrophobic anticancer drug, curcumin, was encapsulated in micelles by using membrane hydration method. The properties of drug loaded micelles were determined by dynamic light scattering, transmission electron microscopy and lower critical solution temperature (LCST) measurements. The micelles size decreases from 102.5 nm for blank micelles to 37.6 nm with 10.8% drug loading, suggesting that the drug plays an important role in the micellization procedure. The LCST decreases from 45.1 °C for blank micelles to 40.6 and 38.3 °C with 5.9 and 10.8% drug loading, respectively. In vitro drug release was performed in pH 7.4 PBS at different temperatures. Data show that the release rate was significantly enhanced above the LCST comparing with that below the LCST. The amount of released drug at 41 °C was ca. 20% higher than that at 37 °C. Burst-like release was depressed due to enhanced interaction between drug with hydrophobic PLA and PMA chains.
Biocompatibility of thermo-responsive PNIPAAm-PLLA-PNIPAAm triblock copolymer as potential drug carrier.
This is custom heading element

ABSTRACT
This work aims to evaluate the cytocompatibility and hemocompatibility of thermo‐responsive polymers as potential drug carrier. Thermo‐responsive poly(N‐isopropyl acrylamide) (PNIPAAm) and poly(N‐isopropyl acrylamide)‐poly(l‐lactide)‐poly(N‐isopropyl acrylamide) (PNIPAAm‐PLLA‐PNIPAAm) triblock copolymer were synthesized by atom transfer radical polymerization using ethyl α‐bromoisobutyrate or Br‐PLLA‐Br as initiator under mild conditions. The self‐assembly and thermo‐responsive properties of the copolymer in aqueous medium were investigated by critical micelle concentration, dynamic light scattering, transmission electron microscopy, and lower critical solution temperature measurements. The critical micelle concentration was 0.014 mg ml−1. Dynamic light scattering and transmission electron microscopy results show that the micelles are spherical in shape with sizes between 20 and 40 nm. The lower critical solution temperature of PNIPAAm and PNIPAAm‐PLLA‐PNIPAAm is 34.8°C and 32.8°C, respectively. 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide assay was carried out to evaluate the cytotoxicity of polymers, and the hemocompatibility was assessed from hemolysis ratio and plasma recalcification time measurements. The results show that PNIPAAm‐PLLA‐PNIPAAm presents outstanding biocompatibility and could be promising for applications in targeted drug delivery.
Hydrogels:
Robust, fast gelling and tunable degradable hydrogels for drug delivery
About the project:
In this project we synthesize hydrogels based on degradable polymers. Our aim is to improve gelation times and/or gel mechanical properties by either adjusting the copolymers compositions/architectures, or by introducing moeities that ensure fast gelations.
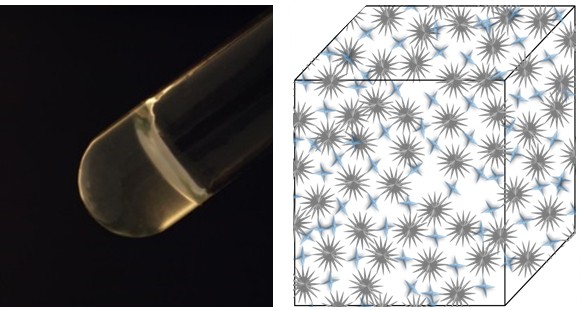
Contact:
Students:

Collaborations:
–
Funding:
Marie Skłodowska-Curie grant
Ultrafast in situ forming poly(ethylene glycol)-poly(amido amine) hydrogels with tunable drug release properties via controllable degradation rates
This is custom heading element
Eur. J. Pharm. Biopharm. 139, 232-239 (2019)
Buwalda S., Bethry A., Hunger S., Kandoussi S., Coudane J., Nottelet B.
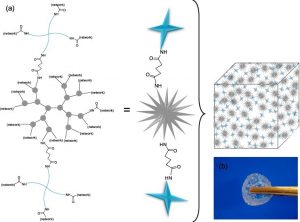
ABSTRACT
Fast in situ forming, chemically crosslinked hydrogels were prepared by the amidation reaction between N-succinimidyl ester end groups of multi-armed poly(ethylene glycol) (PEG) and amino surface groups of poly(amido amine) (PAMAM) dendrimer generation 2.0. To control the properties of the PEG/PAMAM hydrogels, PEGs were used with different arm numbers (4 or 8) as well as different linkers (amide or ester) between the PEG arms and their terminal N-succinimidyl ester groups. Oscillatory rheology measurements showed that the hydrogels form within seconds after mixing the PEG and PAMAM precursor solutions. The storage moduli increased with crosslink density and reached values up to 2.3 kPa for hydrogels based on 4-armed PEG. Gravimetrical degradation experiments demonstrated that hydrogels with ester linkages between PEG and PAMAM degrade within 2 days, whereas amide-linked hydrogels were stable for several months. The release of two different model drugs (fluorescein isothiocyanate-dextran with molecular weights of 4·103 and 2·106 g/mol, FITC-DEX4K and FITC-DEX2000K, respectively) from amide-linked hydrogels was characterized by an initial burst followed by diffusion-controlled release, of which the rate depended on the size of the drug. In contrast, the release of FITC-DEX2000K from ester-containing hydrogels was governed mainly by degradation of the hydrogels and could be modulated via the ratio between ester and amide linkages. In vitro cytotoxicity experiments indicated that the PEG/PAMAM hydrogels are non-toxic to mouse fibroblasts. These in situ forming PEG/PAMAM hydrogels can be tuned with a broad range of mechanical, degradation and release properties and therefore hold promise as a platform for the delivery of therapeutic agents.
Robust & thermosensitive poly(ethylene glycol)-poly(e-caprolactone) star block copolymer hydrogels
This is custom heading element
Polym. Degrad. Stabil. 137, 173–183 (2017)
Buwalda, S. J., Nottelet, B. & Coudane, J.
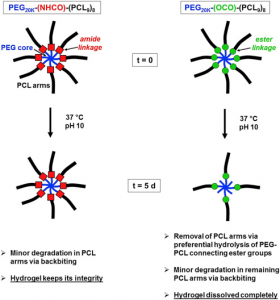
ABSTRACT
Novel 8-armed poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) star block copolymers, possessing an amide or an ester group between the PEG core and the PCL arms (PEG20K-(NHCO)-(PCL9)8 and PEG20K-(OCO)-(PCL9)8), are synthesized by ring opening polymerization of ε-caprolactone in toluene at 110 °C initiated by 8-armed star PEG20K-(NH2)8 and PEG20K-(OH)8, respectively. Compared to linear triblock copolymers with a similar hydrophilic/hydrophobic balance and molecular weight, star block copolymers show better aqueous solubility and yield more homogeneous and transparent hydrogels. PEG20K-(NHCO)-(PCL9)8 hydrogels exhibit a significantly higher storage modulus and in vitro stability in comparison with PEG20K-(OCO)-(PCL9)8 hydrogels of similar concentration and molecular weight. 1H NMR analysis of degrading hydrogel samples clearly demonstrates different degradation mechanisms for the ester and amide type star block copolymers. Their robust mechanical properties, the possibility to be formed in situ and their excellent resistance against hydrolytic degradation make these PEG-PCL star block copolymer hydrogels, especially those based on PEG20K-(NHCO)-(PCL9)8, appealing for various biomedical applications.
Biopolymers based hydrogels for drug delivery
About the project:
In this project the objective is to investigate how modified biopolymers (combined or not with functional polyesters) can lead to improved or even smart gels for the controlled/triggered delivery of therapeutics.
Contact:
Students:


Collaborations:
Dr. D’Este, Dr. Richards, Dr. Moriarty, Dr. Eglin, Dr. Guillaume (AO Research Institute, Switzerland)
Funding:
Marie Skłodowska-Curie grant
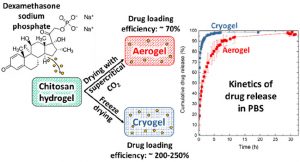
Release kinetics of dexamethasone phosphate from porous chitosan: comparison of aerogels and cryogels
This is custom heading element
Biomacromolecules XXX, XXX (2023)
Coraline Chartier, Sytze Buwalda, Blessing C. Ilochonwu, Hélène Van Den Berghe, Audrey Bethry, Tina Vermonden, Martina Viola, Benjamin Nottelet, Tatiana Budtova
ABSTRACT
Porous chitosan materials as potential wound dressings were prepared via dissolution of chitosan, nonsolvent-induced phase separation in NaOH−water, formation of a hydrogel, and either freeze-drying or supercritical CO2 drying, leading to “cryogels” and “aerogels”, respectively. The hydrophilic drug dexamethasone sodium phosphate was loaded by impregnation of chitosan hydrogel, and the release from cryogel or aerogel was monitored at two pH values relevant for wound healing. The goal was to compare the drug-loading efficiency and release behavior from aerogels and cryogels as a function of the drying method, the materials’ physicochemical properties (density, morphology), and the pH of the release medium. Cryogels exhibited a higher loading efficiency and a faster release in comparison with aerogels. A higher sample density and lower pH value of the release medium resulted in a more sustained release in the case of aerogels. In contrast, for cryogels, the density and pH of the release medium did not noticeably influence release kinetics. The Korsmeyer−Peppas model showed the best fit to describe the release from the porous chitosan materials into the different media.
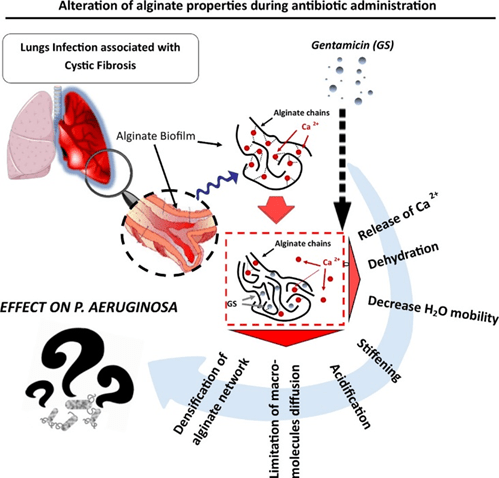
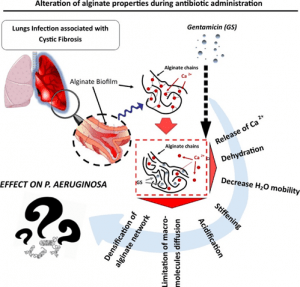
Interaction of gentamicin sulfate with alginate and consequences on the physico-chemical properties of alginate-containing biofilms
This is custom heading element
Int. J. Biol. Macromol. 121, 390–397 (2019)
Heriot, M., Nottelet, B., Garric, X., D’Este, M., Richards, G. R., Moriarty, F. T., Eglin, D. & Guillaume, O.
ABSTRACT
Background: Alginate is one of the main extracellular polymeric substances (EPS) in biofilms of Cystic Fibrosis (CF) patients suffering frompulmonary infections. Gentamicin sulfate (GS) can strongly bind to alginate resulting in loss of pharmacological activity; however neither the mechanism nor its repercussion is fully understood. In this study, we investigated how GS modifies the alginate macromolecular network and its microenvironment. Material and methods: Alginate gels of two different compositions (either enriched in guluronate units (G) or enriched inmannuronate units(M))were crosslinkedwith Ca2+ and exposed to GS at varying times and concentrations.
The complexes formed were characterized via turbidimetry, mechanical tests, swelling assay, calorimetry techniques, nuclear magnetic resonance, Ca2+ displacement, macromolecular probe diffusion and pH alteration.
Results: In presence of GS, the alginate network and its environment undergo a tremendous reorganization in terms of gel density, stiffness, diffusion property, presence and state of the water molecules. We noted that the intensity of those alterations is directly dependent on the polysaccharide motif composition (ratio M/G).
Conclusion: Our results underline the importance of alginate as biofilm component, its pernicious role during antibiotherapy and could represent a potential macromolecular target to improve anti-infectious
therapies.
A method to slow down the ionization-dependent release of risperidone loaded in a thermoresponsive poly(N-acryloyl glycinamide) hydrogel
This is custom heading element
Drug Deliv. Transl. Res. 7, 460–464 (2017)
Boustta, M. & Vert, M.

ABSTRACT
Poly(N-acryloyl glycinamide) polymers are soluble in hot aqueous media that gel rapidly on cooling. This gelatin-like behavior was previously compared with drug delivery requirements. Slow releases were demonstrated in vitro using different model molecules and macromolecules and in vivo using methylene blue. Risperidone is a weak basic drug sparingly soluble in water frequently used to treat patients suffering of schizophrenia. A standard risperidone-poly(N-acryloyl glycinamide) hydrogel formulation was selected from which the drug was allowed to release comparatively in buffered and non-buffered isotonic media at 37 °C under pseudo sink conditions. Linear release was observed in pH = 7.4 phosphate buffer whereas in buffer-free 0.15 M NaCl, the release was initially faster than in the buffer but became rapidly slower as the pH increased from 6.8 to 8.2. These features were related to the ionization-dependent solubility of risperidone. In order to minimize the ionization and thus the solubility of the drug inside the hydrogel despite outside buffering at 7.4, Mg(OH2), a sparingly soluble mineral base, was added to the standard formulation. This addition resulted in a c.a. threefold increase of the zero-order release duration. The method should be applicable to other sparingly soluble weakly basic drugs.


















