Advanced polymeric biomaterials
Hybrid polymeric biomaterials
Peptide/polymers hydrid biomaterials
About the project:
We are working on different types of hybrid materials, including degradable polymers associated with peptides and/or biomacromolecules.
The induction of bioactivity by peptides or natural macromolecules is done in different ways:
– Creation of covalent networks with degradable polymers to obtain bioactive hybrid materials. These networks are micro or nanostructured by electrospinning or 3D printing to obtain nanofibres or more complex shapes for many applications such as soft tissue or meniscus regeneration
– the design of monomers containing peptide sequences and their polymerisation to provide a new class of peptide-based copolymers.
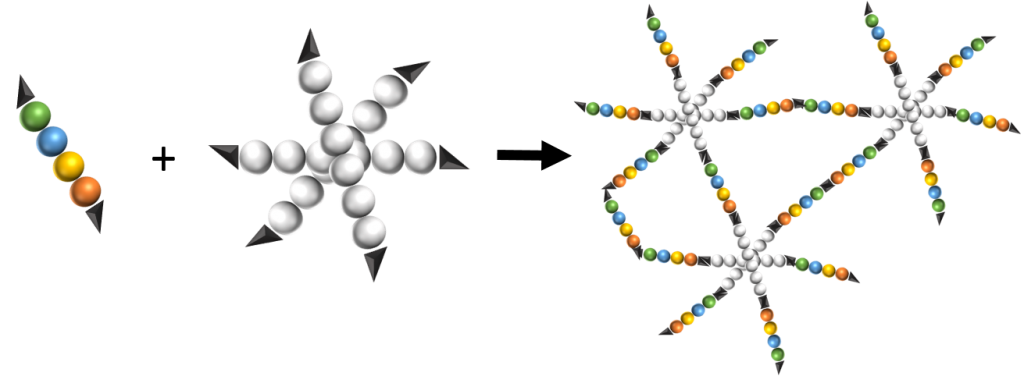
Contact:





Students:


Collaborations:
Pr Subra et Pr Amblard (IBMM-peptides, UMR 5247), Sing Yian Chew (NTU, Singapore)
Funding: –
PhD Program (Doctoral school, University of Montpellier), Algerian Excellence Scholarship
Peptide-guided self-assembly of polyethylene glycol-b-poly(ε-caprolactone-g-peptide) block copolymers
This is custom heading element
Eur. Pol. J. 176, 111386 (2022)
Ayman El Jundi, Matthias Mayor, Enrique Folgado, Chaimaa Gomri, Belkacem Tarek Benkhaled, Arnaud Chaix, Pascal Verdie, Benjamin Nottelet, Mona Semsarilar

ABSTRACT
Biodegradable poly(ethylene glycol)-b-poly(ε-caprolactone-g-peptide) (PEG-b-PCL-g-peptide) copolymers were synthesized using a combination of ring opening polymerization and thiol-yne photoaddition of peptides on the alkyne functional PCL block. The peptides Phe-Phe, Tyr-Tyr and Arg-Gly-Asp were selected based on the expected interactions (Pi-stacking, H-bonding, electrostatic). The self-assembly of these copolymers was studied via testing the effect of various parameters such as the nature of the solvent and non-solvant as well as their ratio,mixing method, temperature and concentration. Structures obtained by varying these parameters were characterised using transmission electron microscopy (TEM) and dynamic light scattering (DLS). Spherical and lamellar structures (oval leaf-shaped) of different sizes were identified as a function of the conditions. The role of the crystallisation and of the peptides was highlighted with more defined and stable structures obtained for Tyr-Tyr functional copolymers.
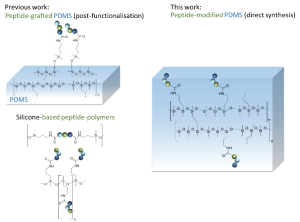
Direct synthesis of peptide-containing silicone. A new way for bioactive materials
This is custom heading element
Chem. Eur. J. 10.1002/chem.202001571
Ahmad Mehdi, Martin Julie, Mohammad Wehbi, Cecile Echalier, Sylvie hunger, Audrey Bethry, Xavier garric, coline Pinese, jean Martinez, Lubomir vezenkov, and gilles subra
ABSTRACT
A simple and efficient way to synthesize peptide-containing silicone materials is described. Silicone oils containing a chosen ratio of bioactive peptide sequences were prepared by acid-catalyzed copolymerization of dichlorodimethylsilane, hybrid dichloromethyl peptidosilane and either Si-vinyl or Si-H functionalized monomers. Functionalized silicone oils were first obtained and then after hydrosilylation cross-linking, bioactive PDMS based materials were straightforward obtained. The introduction of an antibacterial peptide yields PDMS materials showing an interesting activity against Staphylococcus Aureus. In the same way, RGD ligands-containing PDMS demonstrated improved cell adhesion properties. This generic method was fully compatible with the stability of peptides and thus opened the way to the synthesis of a wide range of biologically active silicones.
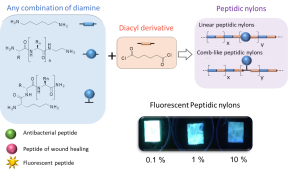
Turning peptides into bioactive nylons
This is custom heading element
Eur. Polym. J. 2020, 135, 109886.
Said Jebors*, Coline Pinese*, Titouan Montheil*, Audrey Bethry, Simon Verquin, Louise Plais, Marie Moulin, Chloé Dupont, Xavier Garric, Ahmad Mehdi, Jean Martinez, Gilles Subra
ABSTRACT
New synthetic textiles with physical and/or biological properties are increasingly used in medical applications. While a simple textile coating is usually carried out to obtain biological properties, covalent grafting should be considered for long-term applications. Herein, we have developed a new hybrid bioactive nylon whose synthesis involves a peptide sequence with a diacyl derivative. Numerous types of peptide-nylons were prepared by varying the molar percentage (0.1 %, 1 % and 10%) and orientation of the peptide in the polymer backbone. Nylons incorporating antibacterial peptides significantly inhibited S. aureus proliferation whereas nylons functionalized with cell-adhesive peptide enhanced the proliferation of L929 fibroblast. These results show that the incorporation of the peptides directly into the nylon skeleton is efficient and provides biological properties that suggest new ways of functionalizing biomedical textiles.
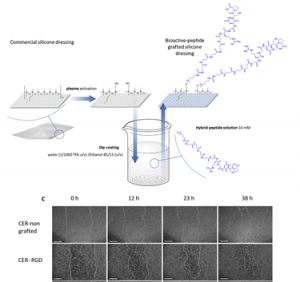
Bioactive peptides grafted silicone dressings: A simple and specific method
This is custom heading element
Materials Today Chemistry 4, 73–83 (2017)
Pinese, C., Jebors, S., Stoebner, P. E., Humblot, V., Verdié, P., Causse, L., Garric, X., Taillades, H., Martinez, J., Mehdi, A. & Subra, G
ABSTRACT
The need for bioactive dressings increases with the population aging and the prevalence of chronic diseases. In contrast, there are very few dressings on the market which are designed to display a chosen bioactivity. In this context, we investigated the surface-functionalization of silicone wound dressing with bioactive peptides. One of the challenges was to avoid multistep grafting reactions involving catalysts, solvents or toxic reagents, which are not suitable for the fabrication of medical devices at an industrial scale. In the other hand, a covalent bonding was necessary to avoid the loss of the biological effect by progressive removal of the peptide in biological fluids generated by the wound. To solve these limitations, we developed a strategy allowing an easy and direct functionalization of silicone. This strategy relies on hybrid silylated bioactive peptides, which chemoselectively react with plasma-activated silicone surfaces. We synthesized three hybrid peptides with wound healing properties, which were grafted on commercially available silicone dressings Cerederm® and Mepitel®. Grafted dressings were evaluated in vitro and enabled a quicker scare recovery and extracellular matrix deposition with human dermal fibroblasts. These results were confirmed by in vivo studies showing an enhanced wound-healing of the pig skin. By this simple method, we transformed inert dressing into bioactive dressing which showed properties of wound healing.
Nanocomposites:
Nanocomposites for bone regeneration
About the project:
Contact:


Students:

Collaborations:
Prof. Combes (CIRIMAT, Toulouse), Prof. Oliva (University of Naples), Prof. Malinconico (IPCB, Naples) Dr. Jérémy Soulié (CIRIMAT, Toulouse)
Funding:
Carnot Balard Cirimat
ANR
Well-defined polyester-grafted silica nanoparticles for biomedical applications: Synthesis and quantitative characterization
Lagarrigue P., Soulié J., Grossin D., Dupret-Bories A., Combes C., Darcos V.
ABSTRACT
Polyester-based composites with silica nanoparticles fillers are promising candidates as biomaterials due to improved mechanical and biological properties. However, nanofillers use generally leads to an inhomogeneous distribution inside the polymer matrix because of agglomeration, decreasing composites overall performances. To improve nanofillers dispersion, the aim of this study is to prepare and characterize poly(D,L lactide) grafted silica nanoparticles using “grafting to” method and to quantify the amount of grafted poly(D,L lactide). Firstly, well-defined N hydroxysuccinimide ester poly(D,L lactide)s were synthetized through a new pathway. Then, amino-functionalized silica nanoparticles were grafted with those customized polyesters yielding an amide covalent bond between both reagents. Such PDLLA grafted nanoparticles were precisely characterized and the grafting amount was quantified using a dual approach based on TGA and FTIR analysis. The synthesis and the characterization methods developed constitute a robust and reproducible way to design well-defined polymer grafted silica nanoparticles that could be used as nanofillers in polymer matrix nanocomposites for biomedical

New synthesis method of HA/P(D,L)LA composites: study of fibronectin adsorption and their effects in osteoblastic behavior for bone tissue engineering
This is custom heading element
Journal of Materials Science: Materials in Medicine (2016), 27(9), 1-10
Yala,S., Boustta,M; Gallet, O; Hindie, M; Carreiras, F; Benachour, H; Sidane, D; Khireddine, H.

ABSTRACT
A novel synthetic method to synthesize hydroxyapatite/poly (D,L) lactic acid biocomposite is presented in this study by mixing only the precursors hydroxyapatite and (D,L) LA monomer without adding neither solvent nor catalyst. Three compns. were successfully synthesized with the wt. ratios of 1/1, 1/3, and 3/5 (hydroxyapatite/(D,L) lactic acid), and the grafting efficiency of poly (D,L) lactic acid on hydroxyapatite surface reaches up to 84 %. SEM and Fourier transform IR spectroscopy showed that the hydroxyapatite particles were successfully incorporated into the poly (D,L) lactic acid polymer and X ray diffraction anal. showed that hydroxyapatite preserved its crystallinity after poly (D,L) lactic acid grafting. Differential scanning calorimetry shows that Tg of hydroxyapatite/poly (D,L) lactic acid composite is less than Tg of pure poly (D,L) lactic acid, which facilitates the shaping of the composite obtained. The addn. of poly (D,L) lactic acid improves the adsorption properties of hydroxyapatite for fibronectin extracellular matrix protein. Furthermore, the presence of poly (D,L) lactic acid on hydroxyapatite surface coated with fibronectin enhanced pre-osteoblast STRO-1 adhesion and cell spreading. These results show the promising potential of hydroxyapatite/poly (D,L) lactic acid composite as a bone substitute material for orthopedic applications and bone tissue engineering.
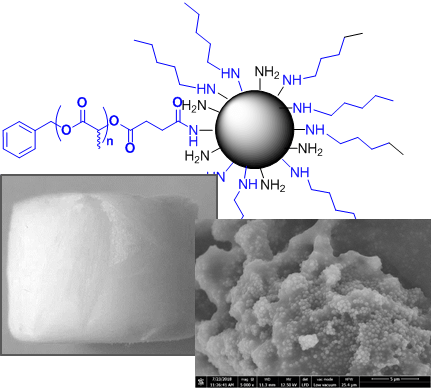
Functionalized PCL/HA nanocomposites as microporous membranes for bone regeneration
This is custom heading element
Mat. Sci. Eng. C-Mater. Biol. Appl. 48, 457–468 (2015)
Basile, M. A., d’Ayala, G. G., Malinconico, M., Laurienzo, P., Coudane, J., Nottelet, B., Della Ragione, F. & Oliva, A.
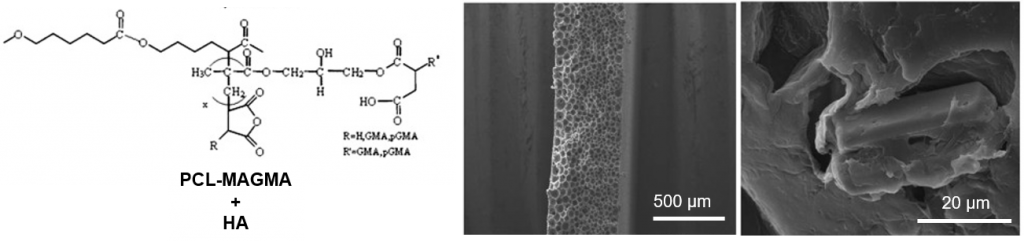
ABSTRACT
In the present work, microporous membranes based on poly(ε-caprolactone) (PCL) and PCL functionalized with amine (PCL-DMAEA) or anhydride groups (PCL-MAGMA) were realized by solvent–non solvent phase inversion and proposed for use in Guided Tissue Regeneration (GTR). Nanowhiskers of hydroxyapatite (HA) were also incorporated in the polymer matrix to realize nanocomposite membranes. Scanning Electron Microscopy (SEM) showed improved interfacial adhesion with HA for functionalized polymers, and highlighted substantial differences in the porosity. A relationship between the developed porous structure of the membrane and the chemical nature of grafted groups was proposed. Compared to virgin PCL, hydrophilicity increases for functionalized PCL, while the addition of HA influences significantly the hydrophilic characteristics only in the case of virgin polymer. A significant increase of in vitro degradation rate was found for PCL-MAGMA based membranes, and at lower extent of PCL-DMAEA membranes. The novel materials were investigated regarding their potential as support for cell growth in bone repair using multipotent mesenchymal stromal cells (MSC) as a model. MSC plated onto the various membranes were analyzed in terms of adhesion, proliferation and osteogenic capacity that resulted to be related to chemical as well as porous structure. In particular, PCL-DMAEA and the relative nanocomposite membranes are the most promising in terms of cell-biomaterial interactions.
Nanocomposites for theranostic and soft/hard tissue interfacing
About the project:
This project aims at combining nanostructured polymers with inorganic particles (eg. SPIONs, dopped hydroxyapatite nanoparticles etc.) in a controlled manner to provide well defined interfaces as well as imaging opportunities.
Contact:

Students:

Collaborations:
Dr. Laurencin, Dr. Guari, Prof. Larionova (IMNO, ICGM UMR 5253), Dr. Lemaire (MINT, Inserm 1066 – CNRS 6021), Dr. Franconi (platform PRISM), Prof. Gilbert (Renssaeler Institute, Troy, USA)
Funding:
Labex Chemisyst, France Life Imafing
Long-term in vivo performances of polylactide / iron oxide nanoparticles core-shell fibrous nanocomposites as MRI-visible magneto-scaffolds
This is custom heading element
Biomat. Sci. 9, 6203–6213 (2021)
Awada H., Seene S., Laurencin D., Lemaire L., Franconi F., Bernex F., Bethry A., Garric X., Guari Y., Nottelet B.
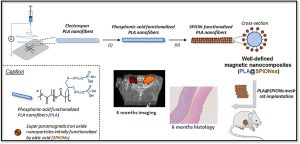
ABSTRACT
There is a growing interest in magnetic nanocomposites in biomaterials science. In particular, nanocomposites that combine poly(lactide) (PLA) nanofibers and super paramagnetic iron oxide nanoparticles (SPIONs), which can be obtained by either electrospinning of a SPIONs suspension in PLA or by precipitating SPIONs at the surface of PLA, are well documented in the literature. However, these two classical processes yield nanocomposites with altered materials properties, and their long-term in vivo fate and performances have in most cases only been evaluated over short periods of time. Recently, we reported a new strategy to prepare well-defined PLA@SPIONs nanofibers with a quasi-monolayer of SPIONs anchored at the surface of PLA electrospun fibers. Herein, we report on a 6-month in vivo rat implantation study with the aim of evaluating the long-term magnetic resonance imaging (MRI) properties of this new class of magnetic nanocomposites, as well as their tissue integration and degradation. Using clinically relevant T2-weighted MRI conditions, we show that the PLA@SPIONs nanocomposites are clearly visible up to 6 months. We also evaluate here by histological analyses the slow degradation of the PLA@SPIONs, as well as their biocompatibility. Overall, these results make these nanocomposites attractive for the development of magnetic biomaterials for biomedical applications.
Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro
This is custom heading element
Acta Biomaterialia 131, 302–313 (2021)
Funnell J.L., Ziemba A.M., Nowak J.F., Awada H., Prokopiou N., Samuel J., Guari Y., Nottelet B., Gilbert R.J.
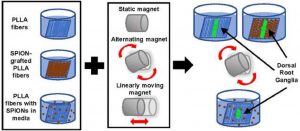
ABSTRACT
Magnetic fiber composites combining superparamagnetic iron oxide nanoparticles (SPIONs) and electrospun fibers have shown promise in tissue engineering fields. Controlled grafting of SPIONs to the fibers post-electrospinning generates biocompatible magnetic composites without altering desired fiber morphology. Here, for the first time, we assess the potential of SPION-grafted scaffolds combined with magnetic fields to promote neurite outgrowth by providing contact guidance from the aligned fibers and mechanical stimulation from the SPIONs in the magnetic field. Neurite outgrowth from primary rat dorsal root ganglia (DRG) was assessed from explants cultured on aligned control and SPION-grafted electrospun fibers as well as on non-grafted fibers with SPIONs dispersed in the culture media. To determine the optimal magnetic field stimulation to promote neurite outgrowth, we generated a static, alternating, and linearly moving magnet and simulated the magnetic flux density at different areas of the scaffold over time. The alternating magnetic field increased neurite length by 40% on control fibers compared to a static magnetic field. Additionally, stimulation with an alternating magnetic field resulted in a 30% increase in neurite length and 62% increase in neurite area on SPION-grafted fibers compared to DRG cultured on PLLA fibers with untethered SPIONs added to the culture media. These findings demonstrate that SPION-grafted fiber composites in combination with magnetic fields are more beneficial for stimulating neurite outgrowth on electrospun fibers than dispersed SPIONs.
Well-defined polyester-grafted silica nanoparticles for biomedical applications: Synthesis and quantitative characterization
Lagarrigue P., Soulié J., Grossin D., Dupret-Bories A., Combes C., Darcos V.
ABSTRACT
Polyester-based composites with silica nanoparticles fillers are promising candidates as biomaterials due to improved mechanical and biological properties. However, nanofillers use generally leads to an inhomogeneous distribution inside the polymer matrix because of agglomeration, decreasing composites overall performances. To improve nanofillers dispersion, the aim of this study is to prepare and characterize poly(D,L lactide) grafted silica nanoparticles using “grafting to” method and to quantify the amount of grafted poly(D,L lactide). Firstly, well-defined N hydroxysuccinimide ester poly(D,L lactide)s were synthetized through a new pathway. Then, amino-functionalized silica nanoparticles were grafted with those customized polyesters yielding an amide covalent bond between both reagents. Such PDLLA grafted nanoparticles were precisely characterized and the grafting amount was quantified using a dual approach based on TGA and FTIR analysis. The synthesis and the characterization methods developed constitute a robust and reproducible way to design well-defined polymer grafted silica nanoparticles that could be used as nanofillers in polymer matrix nanocomposites for biomedical

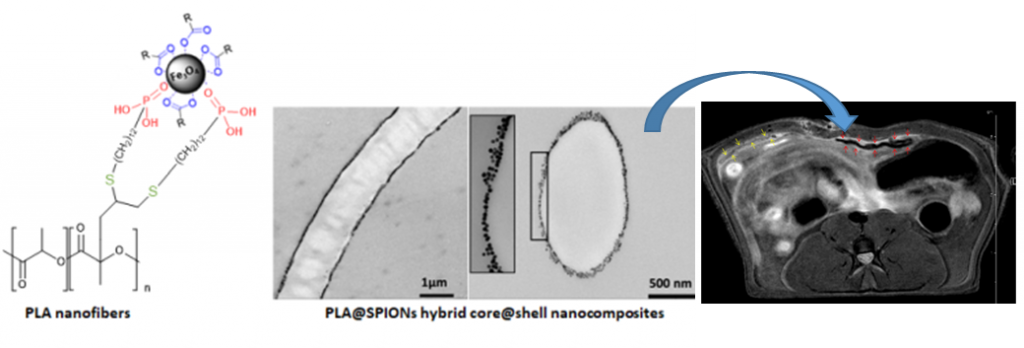
Controlled Anchoring of Iron Oxide Nanoparticles on Polymeric Nanofibers: Easy Access to Core@Shell Organic−Inorganic Nanocomposites for Magneto-Scaffolds
This is custom heading element
ACS Appl. Mater. Interfaces 11, 9519–9529 (2019)
Awada H., Al Samad A., Laurencin D., Gilbert R., Dumail X., El Jundi A., Bethry A., Pomrenke R., Johnson C., Lemaire L., Franconi F., Félix G., Larionova J., Guari Y., Nottelet B.

ABSTRACT
Composites combining superparamagnetic iron oxide nanoparticles (SPIONs) and polymers are largely present in modern (bio)materials. However, while SPIONs embedded in polymer matrices are classically reported, the mechanical and degradation properties of the polymer scaffold are impacted by the SPIONs. Therefore, the controlled anchoring of SPIONs onto polymer surfaces is still a major challenge. Herein, we propose an efficient strategy for the direct and uniform anchoring of SPIONs on the surface of functionalized-polylactide (PLA) nanofibers via a simple free ligand exchange procedure to design PLA@SPIONs core@shell nanocomposites. The resulting PLA@SPIONs hybrid biomaterials are characterized by electron microscopy (SEM and TEM) and EDXS analysis, to probe the morphology and detect elements present at the organic/inorganic interface, respectively. A monolayer of SPIONs with a complete and homogeneous coverage is observed on the surface of PLA nanofibers. Magnetization experiments show that magnetic properties of the nanoparticles are well-preserved after their grafting on the PLA fibers and that the size of the nanoparticles does not change. The absence of cytotoxicity, combined with a high sensitivity of detection in MRI both in vitro and in vivo make these hybrid nanocomposites attractive for the development of magnetic biomaterials for biomedical applications.
Degradable elastomers and biomaterials mechanical behaviours:
Resorbable elastomers for medical applications
About the project:
This project is dedicated to the design and synthesis of biomaterials that exhibit mechanical properties of interest for soft tissue engineering like elastomeric or shape-memory properties.

Contact:




Students:



Collaborations:
Dr. Chagnon & Prof. Favier (TIMC-IMAG, UMR 5525), Dr . David et Dr. Caillol (IAM, ICGM UMR 5253)
Funding:
PhD Program (Doctoral school, University of Montpellier), Financement SATT-AxLR Elastar, ANR Openn.
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet
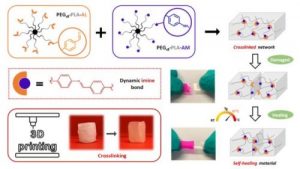
ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet
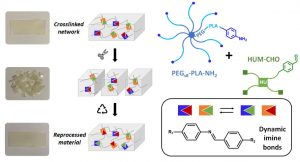
ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet
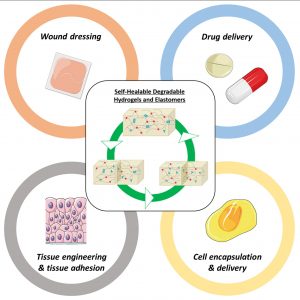
ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Dual-crosslinked degradable elastomeric networks with self-healing properties: bringing multi(catechol) star block copolymers into play
This is custom heading element
ACS Appl. Mater. Interfaces 15, 2077-2091 (2023)
Mathilde Grosjean, Louis Gangolphe, Stéphane Dejean, Sylvie Hunger, Audrey Bethry, Frédéric Bossard, Xavier Garric, Benjamin Nottelet
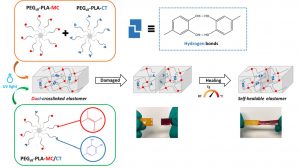
ABSTRACT
In the biomedical field, degradable chemically crosslinked elastomers are interesting materials for tissue engineering applications since they present rubber-like mechanical properties matching with those of soft tissues and are able to preserve their 3D structure over degradation. Their use in biomedical applications requires surgical handling and implantation that can be source of accidental damages responsible for loss of properties. Therefore, their inability to be healed after damage or breaking can be a major drawback. In this work, biodegradable dual-crosslinked networks that exhibit fast and efficient self-healing properties at 37 °C are designed. Self-healable dual-crosslinked (chemically and physically) elastomeric networks are prepared from two methods. The first approach is based on the mix of hydrophobic PEG-PLA star-shaped copolymers functionalized either with catechol or methacrylate moieties. In the second approach, hydrophobic bifunctional PEG-PLA star-shaped copolymers with both catechol and methacrylate on their structure are used. In the two systems the supramolecular network is responsible for the self-healing properties thanks to the dynamic dissociation/re-association of the numerous hydrogen bonds between the catechol groups, whereas the covalent network ensures mechanical properties similar to pure methacrylate networks. The self-healable materials display mechanical properties that are compatible with soft tissues and exhibit linear degradation because of the chemical crosslinks. The performances of networks from mix copolymers vs. bifunctional copolymers are compared and demonstrate the superiority of the later. The biocompatibility of the materials is also demonstrated and confirm the potential of these degradable self-healable elastomeric networks to be used for the design of temporary medical devices.
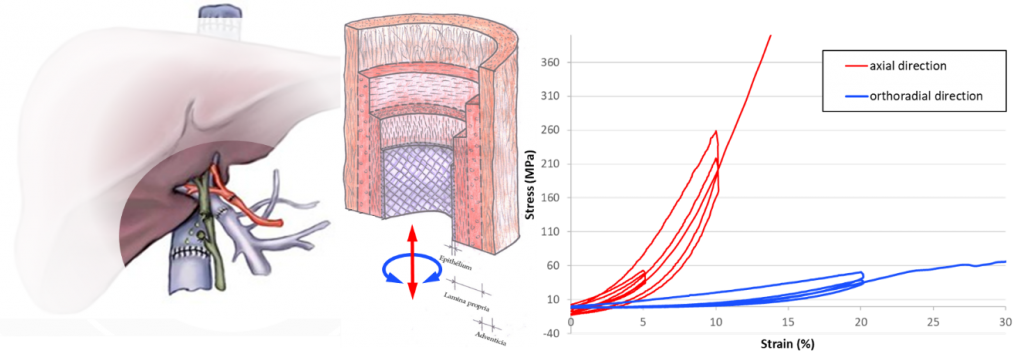
Evaluation of biomaterials mechanical behaviours
About the project:
This project is dedicated to the design and synthesis of biomaterials that exhibit mechanical properties of interest for soft tissue engineering
Contact:




Students:

Collaborations:
Funding:
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet

ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet

ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Dual-crosslinked degradable elastomeric networks with self-healing properties: bringing multi(catechol) star block copolymers into play
This is custom heading element
ACS Appl. Mater. Interfaces 15, 2077-2091 (2023)
Mathilde Grosjean, Louis Gangolphe, Stéphane Dejean, Sylvie Hunger, Audrey Bethry, Frédéric Bossard, Xavier Garric, Benjamin Nottelet

ABSTRACT
In the biomedical field, degradable chemically crosslinked elastomers are interesting materials for tissue engineering applications since they present rubber-like mechanical properties matching with those of soft tissues and are able to preserve their 3D structure over degradation. Their use in biomedical applications requires surgical handling and implantation that can be source of accidental damages responsible for loss of properties. Therefore, their inability to be healed after damage or breaking can be a major drawback. In this work, biodegradable dual-crosslinked networks that exhibit fast and efficient self-healing properties at 37 °C are designed. Self-healable dual-crosslinked (chemically and physically) elastomeric networks are prepared from two methods. The first approach is based on the mix of hydrophobic PEG-PLA star-shaped copolymers functionalized either with catechol or methacrylate moieties. In the second approach, hydrophobic bifunctional PEG-PLA star-shaped copolymers with both catechol and methacrylate on their structure are used. In the two systems the supramolecular network is responsible for the self-healing properties thanks to the dynamic dissociation/re-association of the numerous hydrogen bonds between the catechol groups, whereas the covalent network ensures mechanical properties similar to pure methacrylate networks. The self-healable materials display mechanical properties that are compatible with soft tissues and exhibit linear degradation because of the chemical crosslinks. The performances of networks from mix copolymers vs. bifunctional copolymers are compared and demonstrate the superiority of the later. The biocompatibility of the materials is also demonstrated and confirm the potential of these degradable self-healable elastomeric networks to be used for the design of temporary medical devices.
Smart/active surfaces
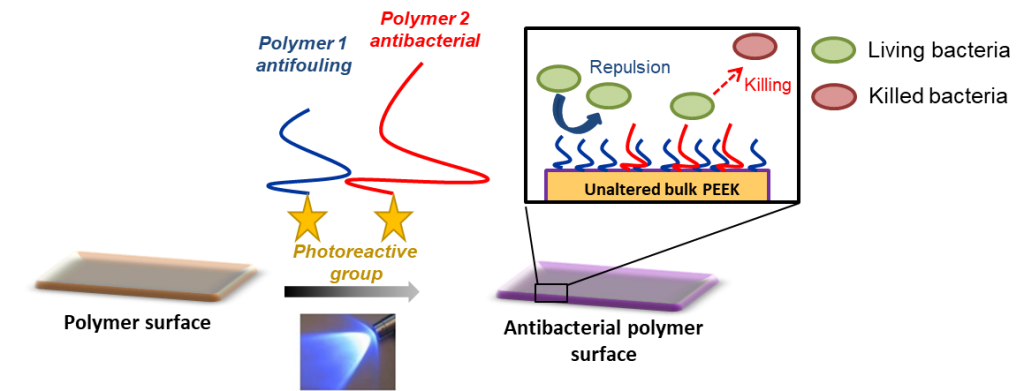
Antibacterial and antifouling surfaces
About the project:
Contact:





Students:
–

Collaborations:
Prof. Lavigne (CHU Nîmes), Prof. Cavallaro (University of Palermo), Dr. Antonio Stocco (L2C, UMR5221), Dr. Moriarty, Dr. Eglin, Dr. Guillaume (AO Research Institute, Switzerland)
Funding:
AO-CMF grant, Campus France, Post-doctoral program UM, MENRT grant (ED 459)
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet

ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet

ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Dual-crosslinked degradable elastomeric networks with self-healing properties: bringing multi(catechol) star block copolymers into play
This is custom heading element
ACS Appl. Mater. Interfaces 15, 2077-2091 (2023)
Mathilde Grosjean, Louis Gangolphe, Stéphane Dejean, Sylvie Hunger, Audrey Bethry, Frédéric Bossard, Xavier Garric, Benjamin Nottelet

ABSTRACT
In the biomedical field, degradable chemically crosslinked elastomers are interesting materials for tissue engineering applications since they present rubber-like mechanical properties matching with those of soft tissues and are able to preserve their 3D structure over degradation. Their use in biomedical applications requires surgical handling and implantation that can be source of accidental damages responsible for loss of properties. Therefore, their inability to be healed after damage or breaking can be a major drawback. In this work, biodegradable dual-crosslinked networks that exhibit fast and efficient self-healing properties at 37 °C are designed. Self-healable dual-crosslinked (chemically and physically) elastomeric networks are prepared from two methods. The first approach is based on the mix of hydrophobic PEG-PLA star-shaped copolymers functionalized either with catechol or methacrylate moieties. In the second approach, hydrophobic bifunctional PEG-PLA star-shaped copolymers with both catechol and methacrylate on their structure are used. In the two systems the supramolecular network is responsible for the self-healing properties thanks to the dynamic dissociation/re-association of the numerous hydrogen bonds between the catechol groups, whereas the covalent network ensures mechanical properties similar to pure methacrylate networks. The self-healable materials display mechanical properties that are compatible with soft tissues and exhibit linear degradation because of the chemical crosslinks. The performances of networks from mix copolymers vs. bifunctional copolymers are compared and demonstrate the superiority of the later. The biocompatibility of the materials is also demonstrated and confirm the potential of these degradable self-healable elastomeric networks to be used for the design of temporary medical devices.
Advanced macromolecular chemistry and architectures:
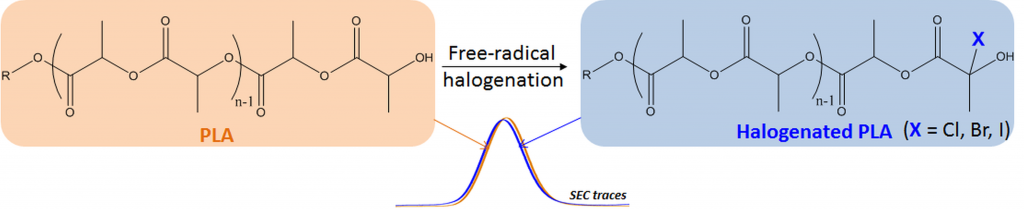
Functional aliphatic polyesters
About the project:
This project aims at exploring various chemical strategies towards (multi)functional polyesters and evaluate their potential in the frame of biomedical applications.
Contact:




Students:
–
Floriane Bahuon
Collaborations:
Dr Lacroix-Desmazes (IAM, ICGM UMR 5253), Roche
Funding:
–
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet

ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet

ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Dual-crosslinked degradable elastomeric networks with self-healing properties: bringing multi(catechol) star block copolymers into play
This is custom heading element
ACS Appl. Mater. Interfaces 15, 2077-2091 (2023)
Mathilde Grosjean, Louis Gangolphe, Stéphane Dejean, Sylvie Hunger, Audrey Bethry, Frédéric Bossard, Xavier Garric, Benjamin Nottelet

ABSTRACT
In the biomedical field, degradable chemically crosslinked elastomers are interesting materials for tissue engineering applications since they present rubber-like mechanical properties matching with those of soft tissues and are able to preserve their 3D structure over degradation. Their use in biomedical applications requires surgical handling and implantation that can be source of accidental damages responsible for loss of properties. Therefore, their inability to be healed after damage or breaking can be a major drawback. In this work, biodegradable dual-crosslinked networks that exhibit fast and efficient self-healing properties at 37 °C are designed. Self-healable dual-crosslinked (chemically and physically) elastomeric networks are prepared from two methods. The first approach is based on the mix of hydrophobic PEG-PLA star-shaped copolymers functionalized either with catechol or methacrylate moieties. In the second approach, hydrophobic bifunctional PEG-PLA star-shaped copolymers with both catechol and methacrylate on their structure are used. In the two systems the supramolecular network is responsible for the self-healing properties thanks to the dynamic dissociation/re-association of the numerous hydrogen bonds between the catechol groups, whereas the covalent network ensures mechanical properties similar to pure methacrylate networks. The self-healable materials display mechanical properties that are compatible with soft tissues and exhibit linear degradation because of the chemical crosslinks. The performances of networks from mix copolymers vs. bifunctional copolymers are compared and demonstrate the superiority of the later. The biocompatibility of the materials is also demonstrated and confirm the potential of these degradable self-healable elastomeric networks to be used for the design of temporary medical devices.
Advanced and well-defined macromolecular architectures
About the project:
Within this project we take advantage of the opportunities offered by advanced macromolecular chemistry to design various macromolecular topologies and evaluate their potential compared to classical linear (co)polymers.
Contact:



Students:

Collaborations:
Dr. Etrych, Dr. Koziolova, Dr. Kostka (Institute of Macromolecular Chemistry, Czech Republic)
Funding:
Campus France
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet

ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet

ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Graft Copolymers with Tunable Amphiphilicity tailored for Efficient Dual Drug Delivery via Encapsulation and pH-sensitive Drug Conjugation
This is custom heading element
Polymer Chemistry 11, 4438–4453 (2020)
Bláhová M., Randárová E., Konefał R., Nottelet B., Etrych T.
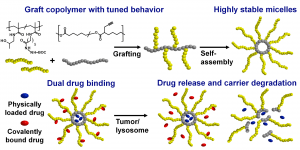
ABSTRACT
Polymer-based drug delivery systems may significantly improve cancer therapy. We developed amphiphilic poly(e-caprolactone)-graft-(poly-N-(2-hydroxypropyl) methacrylamide) copolymers (PCL-graft-pHPMA) with tunable amphiphilicity intended for efficient dual delivery via simultaneous encapsulation of hydrophobic drug, Bcl-2 inhibitor ABT-199, and pH-sensitive conjugation of other chemotherapeutics, doxorubicin, to desired sites, e.g. tumors. Using controlled RAFT polymerization and click chemistry well-defined PCL-graft-pHPMA of diverse Mw and physical properties were prepared. By simple dissolution they self-assembled into highly stable micelles with Dh ≈ 25 nm and low critical micelle concentration (around 5 μg mL-1). The total drug payload reached 17 wt % while maintaining system solubility. The micelles exhibited long-term stability in buffers, while they were cleaved in the presence of lipase, thus proving degradation and drug release after uptake to lysosomes of cancer cells with minimal drug leakage during blood circulation. PCL-graft-pHPMA micelles may serve as a long-circulating drug depo for effective dual therapy of diverse malignancies.
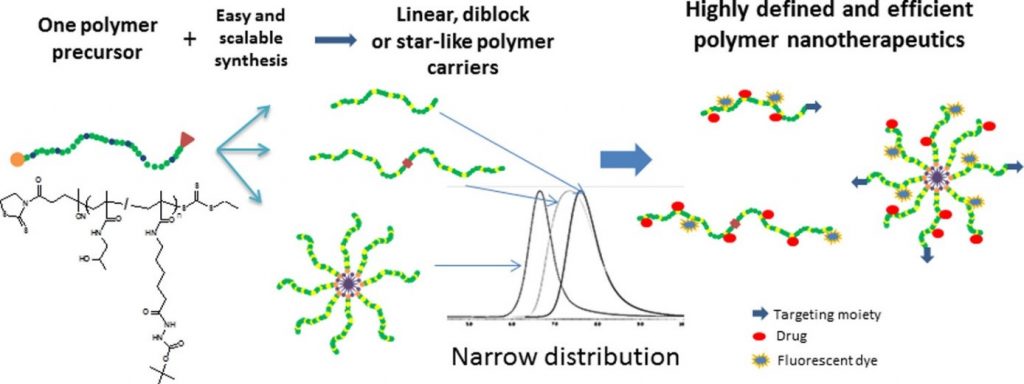
N-(2-Hydroxypropyl)methacrylamide-Based Linear, Diblock, and Starlike Polymer Drug Carriers: Advanced Process for Their Simple Production
This is custom heading element
Biomacromolecules 19, 4003–4013 (2018)
Koziolova, E., Kostka, L., Kotrchova, L., Subr, V., Konefal, R., Nottelet, B. & Etrych, T.
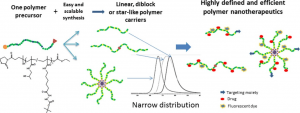
ABSTRACT
We developed a new simplified method for the synthesis of well-defined linear, diblock, or starlike N-(2-hydroxypropyl)methacrylamide (HPMA)-based polymer drug carriers using controlled reversible addition–fragmentation chain transfer polymerization. The prepared monodispersed polymers are after the drug attachment intended for enhanced anticancer therapy. This new approach significantly reduces the number of required synthetic steps and minimizes the consumption of organic solvents during the synthesis. As a result, highly defined linear, diblock, and starlike copolymers designed for pH-triggered drug activation/release in tumor tissue were formed in sufficient amounts for further physicochemical and biological studies. Within the synthesis, we also developed a new procedure for the selective deprotection of tert-butoxycarbonyl hydrazide and amine groups on hydrophilic HPMA copolymers, including the one-pot removal of polymer end groups. We studied and described in detail the kinetics and efficacy of the deprotection reaction. We believe the simplified synthetic approach facilitates the preparation of polymer conjugates bound by the pH-sensitive hydrazone bond and their application in tumor treatment.



