Degradable double hydrophilic block copolymers and tripartite polyionic complex micelles thereof for small interfering ribonucleic acids (siRNA) delivery
Degradable double hydrophilic block copolymers and tripartite polyionic complex micelles thereof for small interfering ribonucleic acids (siRNA) delivery
J. Colloid Interface 580, 449, (2020)
A. El Jundi, M. Morille, N. Bettache, A. Bethry, J. Berthelot, J. Salvador, S. Hunger, Y. Bakkour, E. Belamie, B. Nottelet
ABSTRACT
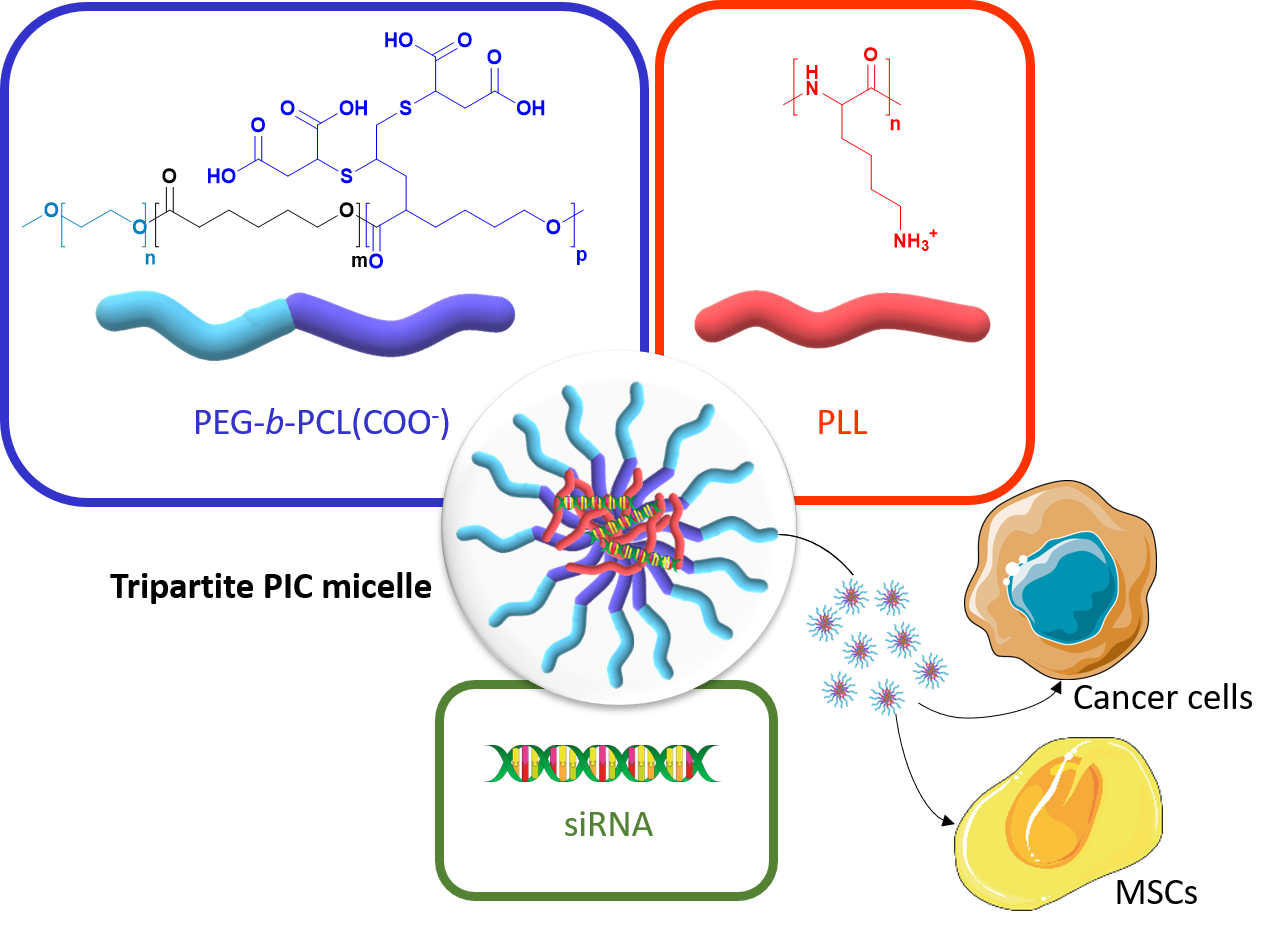
Polymer vectors for gene therapy have been largely investigated as an alternative to viral vectors. In particular, double hydrophilic block copolymers (DHBCs) have shown potential in this domain, but to date studies mainly focus on non-degradable copolymers, which may be a restriction for further development. To overcome this limitation, we synthesized a DHBC (PEG43-b-PCL12(COOH)6.5) composed of a poly(ethylene glycol) (PEG) non-ionic and bioeliminable block and a degradable carboxylic acid-functionalized poly(e-caprolactone) (PCL) block. The potential of this DHBC as an original vector for small interfering ribonucleic acids (siRNA) to formulate tripartite polyionic complex (PIC) micelles with poly(lysine) (PLL) was evaluated. We first studied the impact of the charge ratio (R) on the size and the zeta potential of the resulting micelles. With a charge ratio R=1, one formulation with optimized physico-chemical properties showed the ability to complex 75 % of siRNA. We showed a stability of the micelles at pH 7.4 and a disruption at pH 5, which allowed a pH-triggered siRNA release and proved the pH-stimuli responsive character of the tripartite micelles. In addition, the tripartite PIC micelles were shown to be non-cytotoxic below 40 µg/mL. The potential of these siRNA vectors was further evaluated in vitro: it was found that the tripartite PIC micelles allowed siRNA internalization to be 3 times higher than PLL polyplexes in murine mesenchymal stem cells, and were able to transfect human breast cancer cells. Overall, this set of data pre-validates the use of degradable DHBC as non-viral vectors for the encapsulation and the controlled release of siRNA, which may therefore constitute a sound alternative to non-degradable and/or cytotoxic polycationic vectors.


