Polymer and diagnostics
Polymers for medical imaging
MRI visible polymers for coatings and theranostic applications
About the project:
This project aims at offering chemical strategies that can help making the polymeric implants visible by the clinically relevant MRI procedures.For that, we focus on novel macromolecular MRI contrast agents that can be used as coatings, or as micro- and nanoparticles, and we focus on surface modification to make existing polymers MRI-visible.
Contact:
Students:
Anita Shulz
Mira Younis
Sarah El Habnouni
Collaborations:
Dr. Lemaire (MINT, Inserm 1066 – CNRS 6021), Dr. Franconi (platform PRISM), Prof. Letouzey (CHU Nîmes)
Funding:
Campus France, Post-doctoral program UM, MENRT grant (ED 459), Chercheur d’Avenir 2013
Long-term in vivo performances of polylactide / iron oxide nanoparticles core-shell fibrous nanocomposites as MRI-visible magneto-scaffolds
This is custom heading element
Biomat. Sci. 9, 6203–6213 (2021)
Awada H., Seene S., Laurencin D., Lemaire L., Franconi F., Bernex F., Bethry A., Garric X., Guari Y., Nottelet B.
ABSTRACT
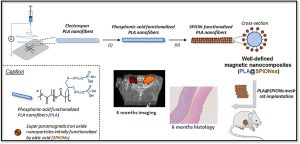
There is a growing interest in magnetic nanocomposites in biomaterials science. In particular, nanocomposites that combine poly(lactide) (PLA) nanofibers and super paramagnetic iron oxide nanoparticles (SPIONs), which can be obtained by either electrospinning of a SPIONs suspension in PLA or by precipitating SPIONs at the surface of PLA, are well documented in the literature. However, these two classical processes yield nanocomposites with altered materials properties, and their long-term in vivo fate and performances have in most cases only been evaluated over short periods of time. Recently, we reported a new strategy to prepare well-defined PLA@SPIONs nanofibers with a quasi-monolayer of SPIONs anchored at the surface of PLA electrospun fibers. Herein, we report on a 6-month in vivo rat implantation study with the aim of evaluating the long-term magnetic resonance imaging (MRI) properties of this new class of magnetic nanocomposites, as well as their tissue integration and degradation. Using clinically relevant T2-weighted MRI conditions, we show that the PLA@SPIONs nanocomposites are clearly visible up to 6 months. We also evaluate here by histological analyses the slow degradation of the PLA@SPIONs, as well as their biocompatibility. Overall, these results make these nanocomposites attractive for the development of magnetic biomaterials for biomedical applications.
Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro
This is custom heading element
Acta Biomaterialia 131, 302–313 (2021)
Funnell J.L., Ziemba A.M., Nowak J.F., Awada H., Prokopiou N., Samuel J., Guari Y., Nottelet B., Gilbert R.J.
ABSTRACT
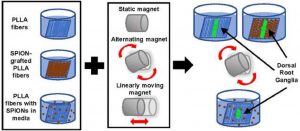
Magnetic fiber composites combining superparamagnetic iron oxide nanoparticles (SPIONs) and electrospun fibers have shown promise in tissue engineering fields. Controlled grafting of SPIONs to the fibers post-electrospinning generates biocompatible magnetic composites without altering desired fiber morphology. Here, for the first time, we assess the potential of SPION-grafted scaffolds combined with magnetic fields to promote neurite outgrowth by providing contact guidance from the aligned fibers and mechanical stimulation from the SPIONs in the magnetic field. Neurite outgrowth from primary rat dorsal root ganglia (DRG) was assessed from explants cultured on aligned control and SPION-grafted electrospun fibers as well as on non-grafted fibers with SPIONs dispersed in the culture media. To determine the optimal magnetic field stimulation to promote neurite outgrowth, we generated a static, alternating, and linearly moving magnet and simulated the magnetic flux density at different areas of the scaffold over time. The alternating magnetic field increased neurite length by 40% on control fibers compared to a static magnetic field. Additionally, stimulation with an alternating magnetic field resulted in a 30% increase in neurite length and 62% increase in neurite area on SPION-grafted fibers compared to DRG cultured on PLLA fibers with untethered SPIONs added to the culture media. These findings demonstrate that SPION-grafted fiber composites in combination with magnetic fields are more beneficial for stimulating neurite outgrowth on electrospun fibers than dispersed SPIONs.
Controlled Anchoring of Iron Oxide Nanoparticles on Polymeric Nanofibers: Easy Access to Core@Shell Organic−Inorganic Nanocomposites for Magneto-Scaffolds
This is custom heading element
ACS Appl. Mater. Interfaces 11, 9519–9529 (2019)
Awada H., Al Samad A., Laurencin D., Gilbert R., Dumail X., El Jundi A., Bethry A., Pomrenke R., Johnson C., Lemaire L., Franconi F., Félix G., Larionova J., Guari Y., Nottelet B.

ABSTRACT
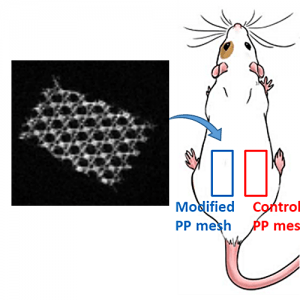
Composites combining superparamagnetic iron oxide nanoparticles (SPIONs) and polymers are largely present in modern (bio)materials. However, while SPIONs embedded in polymer matrices are classically reported, the mechanical and degradation properties of the polymer scaffold are impacted by the SPIONs. Therefore, the controlled anchoring of SPIONs onto polymer surfaces is still a major challenge. Herein, we propose an efficient strategy for the direct and uniform anchoring of SPIONs on the surface of functionalized-polylactide (PLA) nanofibers via a simple free ligand exchange procedure to design PLA@SPIONs core@shell nanocomposites. The resulting PLA@SPIONs hybrid biomaterials are characterized by electron microscopy (SEM and TEM) and EDXS analysis, to probe the morphology and detect elements present at the organic/inorganic interface, respectively. A monolayer of SPIONs with a complete and homogeneous coverage is observed on the surface of PLA nanofibers. Magnetization experiments show that magnetic properties of the nanoparticles are well-preserved after their grafting on the PLA fibers and that the size of the nanoparticles does not change. The absence of cytotoxicity, combined with a high sensitivity of detection in MRI both in vitro and in vivo make these hybrid nanocomposites attractive for the development of magnetic biomaterials for biomedical applications.
UV-triggered photoinsertion of contrast agent onto polymer surfaces for in vivo MRI-visible medical devices
This is custom heading element
Multifunct. Mater. 2 0240012019 (2019)
Schulz A., Lemaire L., Bethry A.; Allegre L., Cardoso M., Bernex F., Franconi F., Goze-Bac C., Taillades H., Garric X., Nottelet B.
ABSTRACT
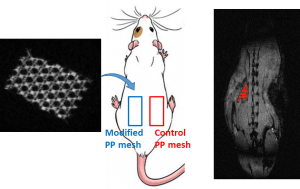
Polymeric materials are largely employed for the manufacturing of implants for various reasons, but they are typically invisible by conventional imaging methods. To improve surgical procedure and postoperative implant follow-up though, biomaterials are needed which allow an accurate and efficient imaging. Here, we present a direct and versatile strategy that allows to covalently immobilize T1 magnetic resonance imaging (MRI) contrast agents at the surface of various clinically relevant polymeric biomaterials. An aryl-azide bearing complex of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and gadolinium (Gd) has been synthesized for easy photografting onto polymer surfaces. Polycaprolactone (PCL), polylactide (PLA), polyurethane (PU), polyetheretherketone (PEEK) and polypropylene (PP) have been selected as clinically relevant substrates and successfully functionalized with the photosensitive MRI probe DOTA/Gd. Following in vitro assessment of their biocompatibility and MRI visibility, commercial MRI-visible PP hernia repair meshes (MRI-meshes) have been prepared. MRI-meshes have been implanted in rats for in vivo evaluation of their imaging capacities over 1 month. Histological evaluation and Gd biodistribution studies have been carried out confirming the potential of this straightforward approach to simply yield imageable medical devices.
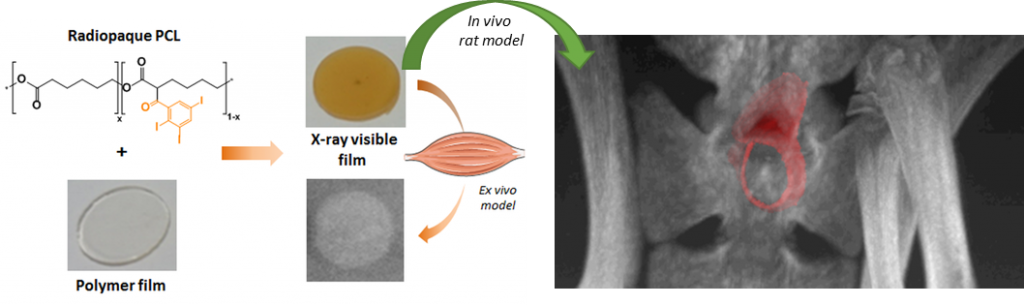
X-ray visible polymers for implantable medical devices
About the project:
This project aims at offering chemical strategies that can help making the polymeric implants visible by the clinically relevant scanner procedures. For that, we focus on novel macromolecular MRI contrast agents that can be used as coatings, or as X-ray radiopaque agent to be formulated in medical devices.
Contact:
Students:
Edouard Girard
Collaborations:
Dr. Rhiele (University of Glasgow)), Dr. Chagnon & Prof .Favier (TIMC-IMAG, UMR 5525)
Funding:
–
From in vitro evaluation to human post-mortem pre-validation of a radiopaque and resorbable internal biliary stent for liver transplantation applications
This is custom heading element
Acta Biomaterialia 106, 66-81, (2020)
Girard E., Chagnon G., Broisat A., Dejean S., Soubies A., Gil H., Sharkawi T., Boucher F. Roth G.S., Trilling B., Nottelet B.
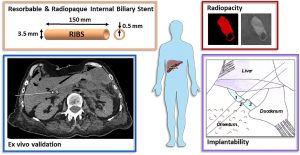
ABSTRACT
The implantation of an internal biliary stent (IBS) during liver transplantation has recently been shown to reduce biliary complications. To avoid a potentially morbid ablation procedure, we developed a resorbable and radiopaque internal biliary stent (RIBS). We studied the mechanical and radiological properties of RIBS upon in vivo implantation in rats and we evaluated RIBS implantability in human anatomical specimens.
For this purpose, a blend of PLA50-PEG-PLA50 triblock copolymer, used as a polymer matrix, and of X-ray-visible triiodobenzoate-poly(e-caprolactone) copolymer (PCL-TIB), as a radiopaque additive, was used to design X-ray-visible RIBS. Samples were implanted in the peritoneal cavity of rats. The radiological, chemical, and biomechanical properties were evaluated during degradation. Further histological studies were carried out to evaluate the degradation and compatibility of the RIBS. A human cadaver implantability study was also performed.
The in vivo results revealed a decline in the RIBS mechanical properties within 3 months, whereas clear and stable X-ray visualization of the RIBS was possible for up to 6 months. Histological analyses confirmed compatibility and resorption of the RIBS, with a limited inflammatory response. The RIBS could be successfully implanted in human anatomic specimens. The results reported in this study will allow the development of trackable and degradable IBS to reduce biliary complications after liver transplantation.
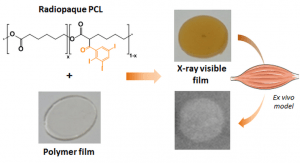
Radiopaque poly(epsilon-caprolactone) as additive for X-ray imaging of temporary implantable medical devices
This is custom heading element
RSC Adv. 5, 84125–84133 (2015)
Samuel, R., Girard, E., Chagnon, G., Dejean, S., Favier, D., Coudane, J. & Nottelet, B
ABSTRACT
In this work we report on the synthesis of two hydrophobic and degradable gadolinium poly(ε-caprolactone) conjugates and their use for the preparation of MRI-visible nanoparticles intended for diagnosis applications. Advantage has been taken from functional poly(ε-caprolactone)s (PCL) bearing propargyl (PCL-yne) or amine groups (P(CL-co-NH2VL)) to yield conjugates by following two strategies. In a first approach, an azido-chelate of gadolinium (Gd(III)) has been conjugated by CuAAC to PCL-yne to yield a polymeric chelate containing 2.6 wt% of Gd(III). In a second approach, a dianhydride Gd(III)-ligand was reacted with P(CL-co-NH2VL) to yield, after complexation with Gd(III) salts, a polymeric chelate containing 15.4 wt% of Gd(III). The polymers biocompatibility was assessed against L929 fibroblasts. In a second part, advantage was taken from the PCLs conjugates hydrophobicity to easily prepare by nanoprecipitation nanoparticles with diameters ranging from 120 to 170 nm. The nanoparticles MRI-visibility was then evaluated and confirmed under the spin-echo and the clinically relevant gradient-echo MRI sequences.
Aliphatic polyesters for medical imaging and theranostic applications
This is custom heading element
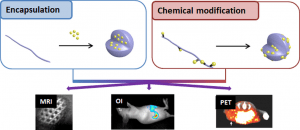
ABSTRACT
Medical imaging is a cornerstone of modern medicine. In that context the development of innovative imaging systems combining biomaterials and contrast agents (CAs)/imaging probes (IPs) for improved diagnostic and theranostic applications focuses intense research efforts. In particular, the classical aliphatic (co)polyesters poly(lactide) (PLA), poly(lactide-co-glycolide) (PLGA) and poly(e-caprolactone) (PCL), attract much attention due to their long track record in the medical field. This review aims therefore at providing a state-of-the-art of polyester-based imaging systems. In a first section a rapid description of the various imaging modalities, including magnetic resonance imaging (MRI), optical imaging, computed tomography (CT), ultrasound (US) and radionuclide imaging (SPECT, PET) will be given. Then, the two main strategies used to combine the CAs/IPs and the polyesters will be discussed. In more details we will first present the strategies relying on CAs/IPs encapsulation in nanoparticles, micelles, dendrimers or capsules. We will then present chemical modifications of polyesters backbones and/or polyester surfaces to yield macromolecular imaging agents. Finally, opportunities offered by these innovative systems will be illustrated with some recent examples in the fields of cell labeling, diagnostic or theranostic applications and medical devices.
Polymers for in vitro diagnostics
Project Biosensors
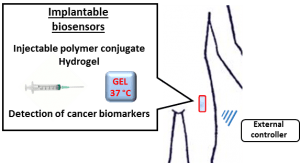
About the project:
Implantable biosensors for cancer biomarkers detection. The project is focused on the development of implantable biosensors based on polymer-peptide conjugate for cancer biomarkers detection.
Contact:
Students:
Collaborations:
Dr May Morris (Institut des Biomolécules Max Mousseron), Pr. Pascal Pujol (CHU Montpellier)
Funding:
Key Initiative MUSE « Biomarkers & Therapy », Université de Montpellier
Congratulations to Jean Coudane for the GFP 2024 honorary award
This is custom heading element

Congratulation Jean!
Your investment in the community and your scientific contribution were rewarded by the GFP 2024, which awarded you the honorary prize!
Congratulations to Anissa Benkhedim for winning the Best student Poster Award
This is custom heading element

Congratulation Anissa!
Your work and the presentation of your poster were rewarded by the “Polymers for a safe and sustainable Future” conference.
Congratulations for your prize : best poster award!
Congratulations to Hélène Van den Berghe for obtaining the HDR
This is custom heading element

We are proud to celebrate an important moment in the life of our team. Today, we would like to extend our warmest congratulations to Hélène Van den Berghe, assistant professor at University of Montpellier on obtaining the HDR. We wish you the best for the rest of your carrier.
Bravo!
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet
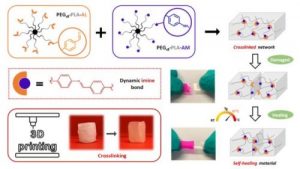
ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.









