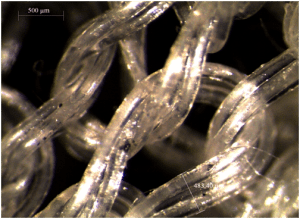
Tissue engineering and medical devices
Biomaterials for tissue engineering
Hyperelastic and absorbable architected biomaterials dedicated to soft tissue reconstruction.
About the project:
This objective of this project is to combine selected degradable polymers and the electrospinning process to produce architected scaffolds to be used in soft tissue engineering. The prediction of the degradation rates, of the evolution of the scaffolds mechanical properties, and of the cells/scaffolds construct behaviour are also forseen.

Contact:
Students:

Collaborations:
Funding:
Laboratoire URGO
Congratulations to Jean Coudane for the GFP 2024 honorary award
This is custom heading element

Congratulation Jean!
Your investment in the community and your scientific contribution were rewarded by the GFP 2024, which awarded you the honorary prize!
Congratulations to Anissa Benkhedim for winning the Best student Poster Award
This is custom heading element

Congratulation Anissa!
Your work and the presentation of your poster were rewarded by the “Polymers for a safe and sustainable Future” conference.
Congratulations for your prize : best poster award!
Congratulations to Hélène Van den Berghe for obtaining the HDR
This is custom heading element

We are proud to celebrate an important moment in the life of our team. Today, we would like to extend our warmest congratulations to Hélène Van den Berghe, assistant professor at University of Montpellier on obtaining the HDR. We wish you the best for the rest of your carrier.
Bravo!
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet
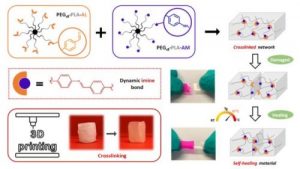
ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Medical devices:
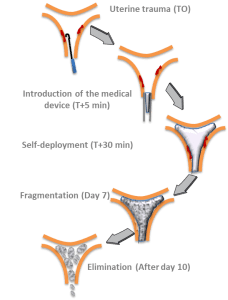
Anti-adhesion degradable medical device
About the project:
We are working mainly on two axes:
Anti-adhesion, self expanding, degradable medical device for the prevention of intra-uterine adhesions.
Anti-adhesion and degradable medical device for the prevention of post-operative adhesions in orthopedic surgery
Contact:
Students:
Salomé Leprince
Stéphanie Huberlant
Lucie Allegre
Collaborations:
Service de Gynécologie Obstétrique (CHU Nîmes)
Pr Michel Chammas (Orthopaedic Surgery Service, CHU Montpellier)
Womed
Funding:
SATT AxLR, Région Occitanie, CHU Nîmes, Université de Montpellier
Algerian Government Excellence grant
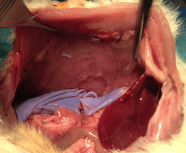
A new bioabsorbable polymer film to prevent peritoneal adhesions validated in a post-surgical animal model
This is custom heading element
Allegre, L., Le Teuff, I., Leprince, S., Warembourg, S., Taillades, H., Garric, X., Letouzey, V. & Huberlant, S.
ABSTRACT
Background – Peritoneal adhesions are a serious surgical postoperative complication. The aim of this study is to investigate, in a rat model, the anti-adhesive effects of a bioabsorbable film of polymer combining polyethylene glycol and polylactic acid that can be easily applied during surgery.
Materials and Methods – Sixty three animals were randomized into five groups according to the anti- adhesion treatment: Hyalobarrier®, Seprafilm®, Polymer A (PA), Polymer B (PB), and control. The rats were euthanized on days 5 and 12 to evaluate the extent, severity and degree of adhesions and histopathological changes. Three animals were euthanized at day 2 in PA, PB and control groups to observe the in vivo elimination.
Results – Macroscopic adhesion formation was significantly lower in the PA group than in the control group at day 5 (median adhesion score 0±0 vs 9.6 ±0.5 p=0.002) and at day 12 (0±0 vs 7.3±4 p=0.02). Furthermore, median adhesion score at day 5 was significantly lower in the PA group than in the Seprafilm® group (0±0 vs 4.2± 3.9 p = 0.03). Residence time of PA seems longer than PB.
Conclusion – The PA bioabsorbable film seems efficient in preventing the formation of peritoneal adhesions
Advanced wound dressings
About the project:
This project gathers different approaches towards original and/or advanced wound dressings. It is based on the combination of 1) degradable polymers exhibiting controlled degradation rates and mechanical properties, 2) chemical modifications and 3) 3D printing techniques or electrospinning to design innovative wound dressings.
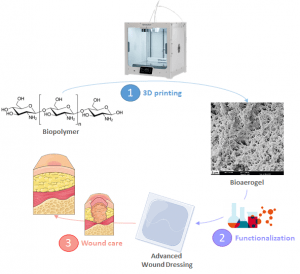
Contact:
Students:


Collaborations:
Laboratoire URGO
Pr. Tatiana Budtova and Dr. Sytze Buwalda (CEMEF, Mines Paristech)
Funding:
CNRS interdisciplinary PhD program
Congratulations to Jean Coudane for the GFP 2024 honorary award
This is custom heading element

Congratulation Jean!
Your investment in the community and your scientific contribution were rewarded by the GFP 2024, which awarded you the honorary prize!
Congratulations to Anissa Benkhedim for winning the Best student Poster Award
This is custom heading element

Congratulation Anissa!
Your work and the presentation of your poster were rewarded by the “Polymers for a safe and sustainable Future” conference.
Congratulations for your prize : best poster award!
Congratulations to Hélène Van den Berghe for obtaining the HDR
This is custom heading element

We are proud to celebrate an important moment in the life of our team. Today, we would like to extend our warmest congratulations to Hélène Van den Berghe, assistant professor at University of Montpellier on obtaining the HDR. We wish you the best for the rest of your carrier.
Bravo!
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet
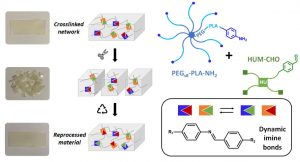
ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet
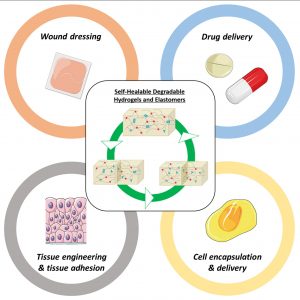
ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Mechanical Evaluation of Hydrogel–Elastomer Interfaces Generated through Thiol–Ene Coupling
This is custom heading element
ACS Appl. Polym. Mater 5, 1364-1373 (2023)
Khai D. Q. Nguyen, Stéphane Dejean, Benjamin Nottelet, Julien E. Gautrot
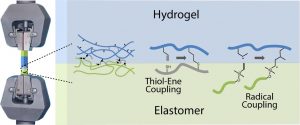
ABSTRACT
The formation of hybrid hydrogel–elastomer scaffolds is an attractive strategy for the formation of tissue engineering constructs and microfabricated platforms for advanced in vitro models. The emergence of thiol–ene coupling, in particular radical-based, for the engineering of cell-instructive hydrogels and the design of elastomers raises the possibility of mechanically integrating these structures without relying on the introduction of additional chemical moieties. However, the bonding of hydrogels (thiol–ene radical or more classic acrylate/methacrylate radical-based) to thiol–ene elastomers and alkene-functional elastomers has not been characterized in detail. In this study, we quantify the tensile mechanical properties of hybrid hydrogel samples formed of two elastomers bonded to a hydrogel material. We examine the impact of radical thiol–ene coupling on the crosslinking of both elastomers (silicone or polyesters) and hydrogels (based on thiol–ene crosslinking or diacrylate chemistry) and on the mechanics and failure behavior of the resulting hybrids. This study demonstrates the strong bonding of thiol–ene hydrogels to alkene-presenting elastomers with a range of chemistries, including silicones and polyesters. Overall, thiol–ene coupling appears as an attractive tool for the generation of strong, mechanically integrated, hybrid structures for a broad range of applications.
Release kinetics of dexamethasone phosphate from porous chitosan: comparison of aerogels and cryogels
This is custom heading element
Biomacromolecules XXX, XXX (2023)
Coraline Chartier, Sytze Buwalda, Blessing C. Ilochonwu, Hélène Van Den Berghe, Audrey Bethry, Tina Vermonden, Martina Viola, Benjamin Nottelet, Tatiana Budtova
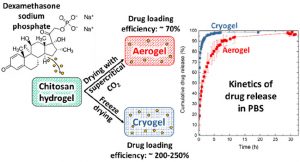
ABSTRACT
Porous chitosan materials as potential wound dressings were prepared via dissolution of chitosan, nonsolvent-induced phase separation in NaOH−water, formation of a hydrogel, and either freeze-drying or supercritical CO2 drying, leading to “cryogels” and “aerogels”, respectively. The hydrophilic drug dexamethasone sodium phosphate was loaded by impregnation of chitosan hydrogel, and the release from cryogel or aerogel was monitored at two pH values relevant for wound healing. The goal was to compare the drug-loading efficiency and release behavior from aerogels and cryogels as a function of the drying method, the materials’ physicochemical properties (density, morphology), and the pH of the release medium. Cryogels exhibited a higher loading efficiency and a faster release in comparison with aerogels. A higher sample density and lower pH value of the release medium resulted in a more sustained release in the case of aerogels. In contrast, for cryogels, the density and pH of the release medium did not noticeably influence release kinetics. The Korsmeyer−Peppas model showed the best fit to describe the release from the porous chitosan materials into the different media.
Development of hybrid bioactive nano fibers composed of star Poly (lactic acid ) and gelatin by sol – gel crosslinking during the electrospinning process
Nanotechnology 34 (2023) 485701
Karima Belabbes, Matthieu Simon, Christopher Yusef Leon-Valdivieso, Mathilde Massonié, Audrey Bethry, Gilles Subra, Xavier Garric and Coline Pinese
ABSTRACT
The design of a biomimetic scaffold is a major challenge in tissue engineering to promote tissue reconstruction. The use of synthetic polymer nano fi bers is widely described as they provide biocompatible matrices whose topography mimics natural extracellular matrix ( ECM ) . To closely match the biochemical composition of the ECM, bioactive molecules such as gelatin are added to the nano fi bers to enhance cell adhesion and proliferation. To overcome the rapid solubilization of gelatin in biological fl uids and to allow a lasting biological effect, the covalent crosslinking of this macromolecule in the network is crucial. The sol – gel route offers the possibility of gentle crosslinking during shaping but is rarely combined with electrospinning. In this study, we present the creation of Poly ( lactic acid )/ Gelatin hybrid nano fi bers by sol – gel route during electrospinning. To enable sol – gel crosslinking, we synthesized star-shaped PLA and functionalized it with silane groups; then we functionalized gelatin with the same groups for their subsequent reaction with the polymer and thus the creation of the hybrid nanonetwork. We evaluated the impact of the presence of gelatin in Poly ( lactic acid )/ Gelatin hybrid nano fi bers at different percentages on the mechanical properties, nanonetwork crosslinking, degradation and biological properties of the hybrid nano fi bers. The addition of gelatin modulated nanonetwork crosslinking that impacted the stiffness of the nano fi bers, resulting in softer materials for the cells. Moreover, these hybrid nano fi bers also showed a signi fi cant improvement in fi broblast proliferation and present a degradation rate suitable for tissue reconstruction. Finally, the bioactive hybrid nano fi bers possess versatile properties, interesting for various potential applications in tissue reconstruction.
Keywords: silylated star PLA, silylated gelatin, hybrid 3D network, bioactive scaffolds, tissue reconstruction
Implantable medical devices for soft tissues
About the project:
We design polymers to improve or create new implants in the field of soft tissue regeneration (ligament prosthesis, hernia…)
Contact:
Students:
Collaborations:
Société Biom’up (CHU Montpellier), Dr Danièle Noël (IRMB, U1183, Montpellier)
Funding:
Industrial grant CIFRE Biom’up, MENRT grant (ED CBS2)
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet

ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Dual-crosslinked degradable elastomeric networks with self-healing properties: bringing multi(catechol) star block copolymers into play
This is custom heading element
ACS Appl. Mater. Interfaces 15, 2077-2091 (2023)
Mathilde Grosjean, Louis Gangolphe, Stéphane Dejean, Sylvie Hunger, Audrey Bethry, Frédéric Bossard, Xavier Garric, Benjamin Nottelet
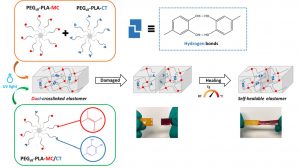
ABSTRACT
In the biomedical field, degradable chemically crosslinked elastomers are interesting materials for tissue engineering applications since they present rubber-like mechanical properties matching with those of soft tissues and are able to preserve their 3D structure over degradation. Their use in biomedical applications requires surgical handling and implantation that can be source of accidental damages responsible for loss of properties. Therefore, their inability to be healed after damage or breaking can be a major drawback. In this work, biodegradable dual-crosslinked networks that exhibit fast and efficient self-healing properties at 37 °C are designed. Self-healable dual-crosslinked (chemically and physically) elastomeric networks are prepared from two methods. The first approach is based on the mix of hydrophobic PEG-PLA star-shaped copolymers functionalized either with catechol or methacrylate moieties. In the second approach, hydrophobic bifunctional PEG-PLA star-shaped copolymers with both catechol and methacrylate on their structure are used. In the two systems the supramolecular network is responsible for the self-healing properties thanks to the dynamic dissociation/re-association of the numerous hydrogen bonds between the catechol groups, whereas the covalent network ensures mechanical properties similar to pure methacrylate networks. The self-healable materials display mechanical properties that are compatible with soft tissues and exhibit linear degradation because of the chemical crosslinks. The performances of networks from mix copolymers vs. bifunctional copolymers are compared and demonstrate the superiority of the later. The biocompatibility of the materials is also demonstrated and confirm the potential of these degradable self-healable elastomeric networks to be used for the design of temporary medical devices.
Degradable Bioadhesives Based on Star PEG–PLA Hydrogels for Soft Tissue Applications
This is custom heading element
Biomacromolecules XX, XX (2022)
Mathilde Grosjean, Edouard Girard, Audrey Bethry, Grégory Chagnon, Xavier Garric, Benjamin Nottelet
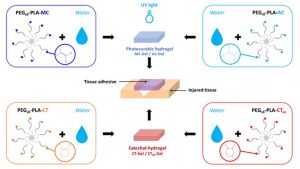
ABSTRACT
Tissue adhesives are interesting materials for wound treatment as they present numerous advantages compared to traditional methods of wound closure such as suturing and stapling. Nowadays, fibrin and cyanoacrylate glues are the most widespread commercial biomedical adhesives, but these systems display some drawbacks. In this study, degradable bioadhesives based on PEG–PLA star-shaped hydrogels are designed. Acrylate, methacrylate, and catechol functional copolymers are synthesized and used to design various bioadhesive hydrogels. Various types of mechanisms responsible for adhesion are investigated (physical entanglement and interlocking, physical interactions, chemical bonds), and the adhesive properties of the different systems are first studied on a gelatin model and compared to fibrin and cyanoacrylate references. Hydrogels based on acrylate and methacrylate reached adhesion strength close to cyanoacrylate (332 kPa) with values of 343 and 293 kPa, respectively, whereas catechol systems displayed higher values (11 and 19 kPa) compared to fibrin glue (7 kPa). Bioadhesives were then tested on mouse skin and human cadaveric colonic tissue. The results on mouse skin confirmed the potential of acrylate and methacrylate gels with adhesion strength close to commercial glues (15–30 kPa), whereas none of the systems led to high levels of adhesion on the colon. These data confirm that we designed a family of degradable bioadhesives with adhesion strength in the range of commercial glues. The low level of cytotoxicity of these materials is also demonstrated and confirm the potential of these hydrogels to be used as surgical adhesives.
Bioresorbable bilayered elastomers/hydrogels constructs with gradual interfaces for the fast actuation of self-rolling tubes
This is custom heading element
ACS Appl. Mater. Interfaces 14, 43719–43731 (2022)
Mathilde Grosjean, Sidzigui Ouedraogo, Stéphane Déjean, Xavier Garric, Valeriy Luchnikov, Arnaud Ponche, Noëlle Mathieu, Karine Anselme, Benjamin Nottelet
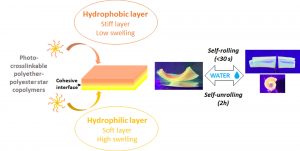
ABSTRACT
In the biomedical field, self-rolling materials provide interesting opportunities to develop medical devices suitable for drug or cell encapsulation. However, to date a major limitation for medical applications is the use of non-biodegradable and non-biocompatible polymers that are often reported for such applications, or the slow actuation witnessed with degradable systems. In this work, biodegradable self-rolling tubes that exhibit a spontaneous and rapid actuation when immersed in water are designed. Photo-crosslinkable hydrophilic and hydrophobic PEG-PLA star-shaped copolymers are prepared and used to prepare bilayered constructs. Thanks to the discrete mechanical and swelling properties of each layer and the cohesive/gradual nature of the interface, the resulting bilayered films are able to self-roll in water in less than 30 seconds depending on the nature of the hydrophilic layer and on the shape of the sample. The cytocompatibility and degradability of the materials are demonstrated and confirm the potential of such self-rolling resorbable biomaterials in the field of temporary medical devices.
Evaluation of a biodegradable PLA–PEG–PLA internal biliary stent for liver transplantation: in vitro degradation and mechanical properties
This is custom heading element
J. Biomed. Mater. Res. 1-10, (2020)
Girard E., Chagnon G., Moreau-Gaudry A., Letoublon C., Favier D., Dejean S., Trilling B., Nottelet B.
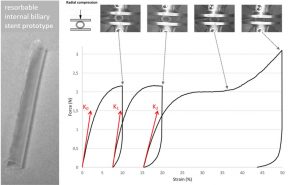
ABSTRACT
Internal biliary stenting during biliary reconstruction in liver transplantation decrease anastomotic biliary complications. Implantation of a resorbable internal biliary stent (RIBS) is interesting since it would avoid an ablation gesture. The objective of present work was to evaluate adequacy of selected PLA-b-PEG-b-PLA copolymers for RIBS aimed to secure biliary anastomose during healing and prevent complications, such as bile leak and stricture. The kinetics of degradation and mechanical properties of a RIBS prototype were evaluated with respect to the main bile duct stenting requirements in liver transplantation. For this purpose, RIBS degradation under biliary mimicking solution versus standard phosphate buffer control solution was discussed. Morphological changes, mass loss, water uptake, molecular weight, permeability, pH variations, and mechanical properties were examined over time. The permeability and mechanical properties were evaluated under simulated biliary conditions to explore the usefulness of a PLA-b-PEG-b-PLA RIBS to secure biliary anastomosis. Results showed no pH influence on the kinetics of degradation, with degradable RIBS remaining impermeable for at least 8 weeks, and keeping its mechanical properties for 10 weeks. Complete degradation is reached at 6 months. PLA-b-PEG-b-PLA RIBS have the required in vitro degradation characteristics to secure biliary anastomosis in liver transplantation and envision in vivo applications
Biomimicking Fiber Platform with Tunable Stiffness to Study Mechanotransduction Reveals Stiffness Enhances Oligodendrocyte Differentiation but Impedes Myelination through YAP‐Dependent Regulation
This is custom heading element
William Ong, Nicolas Marinval, Junquan Lin, Mui Hoon Nai, Yee-Song Chong, Coline Pinese, Sreedharan Sajikumar, Chwee Teck Lim, Charles Ffrench-Constant, Marie E. Bechler, and Sing Yian Chew
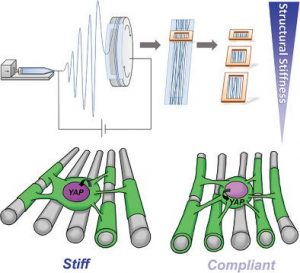
ABSTRACT
A key hallmark of many diseases, especially those in the central nervous system (CNS), is the change in tissue stiffness due to inflammation and scarring. However, how such changes in microenvironment affect the regenerative process remains poorly understood. Here, a biomimicking fiber platform that provides independent variation of fiber structural and intrinsic stiffness is reported. To demonstrate the functionality of these constructs as a mechanotransduction study platform, these substrates are utilized as artificial axons and the effects of axon structural versus intrinsic stiffness on CNS myelination are independently analyzed. While studies have shown that substrate stiffness affects oligodendrocyte differentiation, the effects of mechanical stiffness on the final functional state of oligodendrocyte (i.e., myelination) has not been shown prior to this. Here, it is demonstrated that a stiff mechanical microenvironment impedes oligodendrocyte myelination, independently and distinctively from oligodendrocyte differentiation. Yes-associated protein is identified to be involved in influencing oligodendrocyte myelination through mechanotransduction. The opposing effects on oligodendrocyte differentiation and myelination provide important implications for current work screening for promyelinating drugs, since these efforts have focused mainly on promoting oligodendrocyte differentiation. Thus, the platform may have considerable utility as part of a drug discovery program in identifying molecules that promote both differentiation and myelination.
In Vivo Tissue-Engineered Vascular Grafts
This is custom heading element
Tissue-Engineered Vascular Grafts, Reference Series in
Biomedical Engineering
Walpoth B.H., de Valence S., Tille J-C., Mugnai D., Sologashvili T., Mrówczyński W.,
Cikirikcioglu M., Pektok E., Osorio S., Innocente F., Bochaton-Piallat M-L., Nottelet B., Kalangos A., Gurny R.
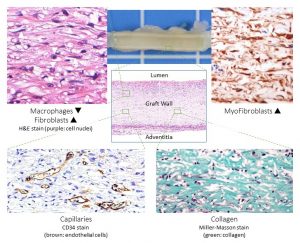
ABSTRACT
Vascular grafts are needed for coronary and peripheral vascular bypass surgeries as well as for access surgeries for hemodialysis and reconstruction of congenital heart defects. Despite good results in the large caliber, small caliber (<6 mm) show unsatisfactory clinical results. Tissue-engineered vascular grafts (TEVG) have been made using several approaches ranging from acellular synthetic or biologic polymer scaffolds to decellularized natural matrices, self-assembled cell-based bioreactor matured, or 3D cell-printed constructs. This chapter will focus mainly on in vivo tissue engineering which was used as first-in-man. This is based on an acellular, synthetic, degradable, polymer scaffold which is repopulated by the host cells after implantation to create a “neo-artery.” Advantages are shelf-readiness; simple, costeffective manufacturing; and avoidance of bioreactor cell maturation. Short-, mid-, and long-term experimental and clinical results show good cellular remodeling with extracellular matrix formation and endothelialization as well as patency and function. Thus, the approach of using an acellular, synthetic, biodegradable scaffold is an optimal clinical option for TEVG.
From in vitro evaluation to human post-mortem pre-validation of a radiopaque and resorbable internal biliary stent for liver transplantation applications
This is custom heading element
Acta Biomaterialia 106, 66-81, (2020)
Girard E., Chagnon G., Broisat A., Dejean S., Soubies A., Gil H., Sharkawi T., Boucher F. Roth G.S., Trilling B., Nottelet B.
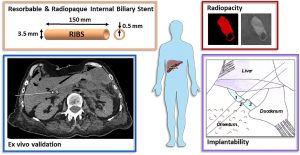
ABSTRACT
The implantation of an internal biliary stent (IBS) during liver transplantation has recently been shown to reduce biliary complications. To avoid a potentially morbid ablation procedure, we developed a resorbable and radiopaque internal biliary stent (RIBS). We studied the mechanical and radiological properties of RIBS upon in vivo implantation in rats and we evaluated RIBS implantability in human anatomical specimens.
For this purpose, a blend of PLA50-PEG-PLA50 triblock copolymer, used as a polymer matrix, and of X-ray-visible triiodobenzoate-poly(e-caprolactone) copolymer (PCL-TIB), as a radiopaque additive, was used to design X-ray-visible RIBS. Samples were implanted in the peritoneal cavity of rats. The radiological, chemical, and biomechanical properties were evaluated during degradation. Further histological studies were carried out to evaluate the degradation and compatibility of the RIBS. A human cadaver implantability study was also performed.
The in vivo results revealed a decline in the RIBS mechanical properties within 3 months, whereas clear and stable X-ray visualization of the RIBS was possible for up to 6 months. Histological analyses confirmed compatibility and resorption of the RIBS, with a limited inflammatory response. The RIBS could be successfully implanted in human anatomic specimens. The results reported in this study will allow the development of trackable and degradable IBS to reduce biliary complications after liver transplantation.
Biomedical innovation
Hydrogels from biocompatible polymers for actinide decontamination
About the project:
Hydrogels from biocompatible polymers for actinide decontamination (DECAP). The DECAP project is focused on the development of innovative hydrogels prepared from polymeric materials for external actinide decontamination. The objective is to prepare new chelating macromolecules able to complex radionuclides with the controlled synthesis of complexing copolymers.
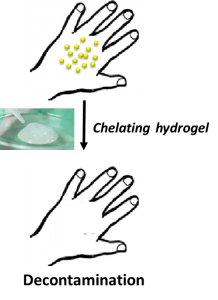
Contact:
Students:

Collaborations:
Florence Agnely (Institut Galien Paris-Sud), Nicolas Dacheux (Institut de Chimie Séparative de Marcoule), Sophie Monge (Institut Charles Gerhardt de Montpellier)
Funding:
ANR ASTRID
Congratulations to Jean Coudane for the GFP 2024 honorary award
This is custom heading element

Congratulation Jean!
Your investment in the community and your scientific contribution were rewarded by the GFP 2024, which awarded you the honorary prize!
Congratulations to Anissa Benkhedim for winning the Best student Poster Award
This is custom heading element

Congratulation Anissa!
Your work and the presentation of your poster were rewarded by the “Polymers for a safe and sustainable Future” conference.
Congratulations for your prize : best poster award!
Congratulations to Hélène Van den Berghe for obtaining the HDR
This is custom heading element

We are proud to celebrate an important moment in the life of our team. Today, we would like to extend our warmest congratulations to Hélène Van den Berghe, assistant professor at University of Montpellier on obtaining the HDR. We wish you the best for the rest of your carrier.
Bravo!
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Implantable medical device for the capture and destruction of cancer cells in vivo.
About the project:
Contact:
Students:

Collaborations:
Dr Jean-Marie Ramirez (IBMM, Montpellier) ; Dr Benoit Charlot (IES, Montpellier)
Funding:
Region Occitanie, SATT AxLR CAPDCM
Congratulations to Jean Coudane for the GFP 2024 honorary award
This is custom heading element

Congratulation Jean!
Your investment in the community and your scientific contribution were rewarded by the GFP 2024, which awarded you the honorary prize!
Congratulations to Anissa Benkhedim for winning the Best student Poster Award
This is custom heading element

Congratulation Anissa!
Your work and the presentation of your poster were rewarded by the “Polymers for a safe and sustainable Future” conference.
Congratulations for your prize : best poster award!
Congratulations to Hélène Van den Berghe for obtaining the HDR
This is custom heading element

We are proud to celebrate an important moment in the life of our team. Today, we would like to extend our warmest congratulations to Hélène Van den Berghe, assistant professor at University of Montpellier on obtaining the HDR. We wish you the best for the rest of your carrier.
Bravo!
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
3D printing and shaping processes for improved medical devices
About the project:

Contact:
Students:


Collaborations:
Funding:
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet

ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet

ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
PLA scaffolds production from Thermally Induced Phase Separation: Effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide
This is custom heading element
Supercrit. Fluids 136, 123–135 (2018)
Gay, S., Lefebvre, G., Bonnin, M., Nottelet, B., Boury, F., Gibaud, A. & Calvignac, B.

ABSTRACT
In this work, a relatively large scale of PLA scaffolds was produced using thermally induced phase separation (TIPS) combined with a supercritical carbon dioxide (SC-CO2) drying step as a green alternative. For the TIPS step, the phase separation of PLA and 1,4-dioxane solvent was controlled by adjusting the process conditions such as the polymer concentration and molecular weight, the 1,4-dioxane solvent power and the cooling conditions. The scaffolds morphology was analyzed by scanning electron microscopy. Their structural and mechanical properties were correlated together with the possibility to tune them by controlling the process conditions. An environmental analysis using the Life Cycle Assessment (LCA) methodology confirmed a reduction of at least 50% of the environmental impact of the whole process using the SC-CO2 drying compared to the traditional freeze-drying technology. This work is the first known attempt to conduct the LCA methodology on TIPS process for the PLA scaffolds production.