Jean Coudane

Jean Coudane
Professor emeritus, Faculty of pharmacy, University of Montpellier
Jean is an alumnus of the ENS Cachan, he has passed aggregation in Chemistry before moving to INSA of Rouen as assistant professor in organic chemistry in the Laboratory of Macromolecular Substances, managed by Professor Maréchal. in 1991 he moved at the Faculty of Pharmacy of Montpellier in the CRBA (Research Center on Artificial Biopolymers) team, managed par Dr Michel Vert. From 2007 to 2018 he managed the “Artificial Biopolymers” department of IBMM (Max Mousseron Institute on Biomolecules). His research skills are mainly dedicated to the chemical modifications of biodegradable and biocompatible (co)polymers, especially (co)polyesters, for applications in Health, such as implantable polymeric medical devices or polymeric drug vectors.
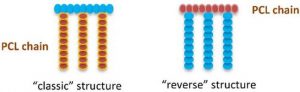
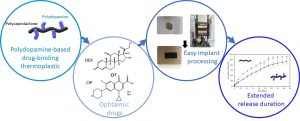
Part of his recent research work is dedicated to the synthesis of advanced biomaterials via the functionalization of biodegradable aliphatic (co)polyesters. The main applications are i) in drug delivery and vectorization (multifunctional polycaprolactone-graft-polysaccharides) especially for anticancer activity ii) medical devices and tissue engineering: core and surface modification for X-Rays and IRM-imaging, antibacterial applications…
Jean is co-author of 122 papers and 11 patents.
Contact:
Jean.coudane(a)umontpellier.fr
+33 (0)4 11 75 97 11
5 publications récentes:
Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic Polyesters for Medical Imaging and Theranostic Applications. European Journal of Pharmaceutics and Biopharmaceutics 2015, 97, 350–370. https://doi.org/10.1016/j.ejpb.2015.06.023.
Samad, A. A.; Bethry, A.; Janouskova, O.; Ciccione, J.; Wenk, C.; Coll, J.-L.; Subra, G.; Etrych, T.; Omar, F. E.; Bakkour, Y.; lterative Photoinduced Chain Functionalization as a Generic Platform for Advanced Polymeric Drug Delivery Systems. Macromolecular Rapid Communications 2018, 39 (3), 1700502. https://doi.org/10.1002/marc.201700502.
Samad, A. A.; Bethry, A.; Koziolová, E.; Netopilík, M.; Etrych, T.; Bakkour, Y.; Coudane, J.; Omar, F. E.; Nottelet, B. PCL–PEG Graft Copolymers with Tunable Amphiphilicity as Efficient Drug Delivery Systems. J. Mater. Chem. B 2016, 4 (37), 6228–6239. https://doi.org/10.1039/C6TB01841F.
Sardo, C.; Nottelet, B.; Triolo, D.; Giammona, G.; Garric, X.; Lavigne, J.-P.; Cavallaro, G.; Coudane, J. When Functionalization of PLA Surfaces Meets Thiol–Yne Photochemistry: Case Study with Antibacterial Polyaspartamide Derivatives. Biomacromolecules 2014, 15 (11), 4351–4362. https://doi.org/10.1021/bm5013772.
Coudane J, Leprince S, Garric X, Paniagua C, Huberlant S, Letouzey V. Composition of diblock and triblock copolymers and the use thereof in the prevention of tissues adhesions. WO2016020613