Stabilization of poly(ethylene glycol)-poly(epsilon-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties
Stabilization of poly(ethylene glycol)-poly(epsilon-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties
Colloid Interface Sci. 514, 468–478 (2018)
Buwalda, S., Al Samad, A., El Jundi, A., Bethry, A., Bakkour, Y., Coudane, J. & Nottelet, B
ABSTRACT
Hypothesis
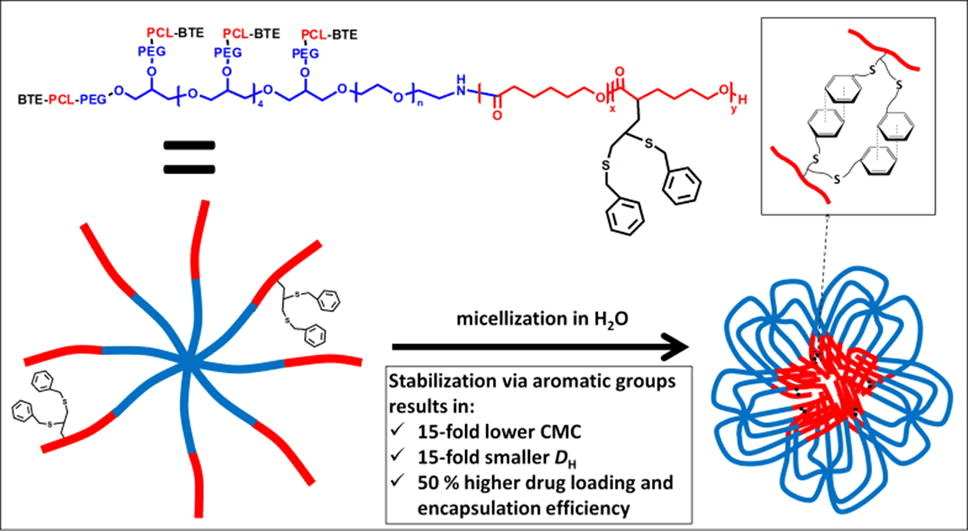
The functionalization of poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) block copolymers with moieties allowing for core-crosslinking is expected to result in improved micellar stability and drug delivery properties.
Experiments
PEG-(PCL)8 star block copolymers were functionalized with pendant benzylthioether (BTE) groups by applying an anionic post-polymerization modification technique followed by photoradical thiol-yne addition of benzyl mercaptan. The micellar properties of PEG-(PCL)8 and PEG-(PCL-BTE)8 were studied and compared in terms of critical micelle concentration (CMC), size, morphology, drug loading and release and in vitro cytotoxicity.
Findings
In comparison with unmodified PEG-(PCL)8 micelles, PEG-(PCL-BTE)8 micelles exhibited a 15-fold lower CMC, a 15-fold smaller size and a 50% higher drug loading and encapsulation efficiency thanks to the presence of pendant benzyl groups which provide the possibility for micellar core-crosslinking via supramolecular π-π stacking and additional hydrophobic interactions. Whereas the PEG-(PCL)8 micelles showed significant aggregation during in vitro cytotoxicity experiments, the PEG-(PCL-BTE)8 micelles showed no signs of aggregation and were capable of solubilizing high concentrations of curcumin, resulting in a significant decrease in MCF-7 cell viability after 48 h. Their ease of synthesis combined with promising results regarding drug delivery make the PEG-(PCL-BTE)8 micelles appealing for application in the field of encapsulation.


