Vincent Darcos

Vincent Darcos
Research engineer, Faculty of pharmacy, University of Montpellier
Vincent is a CNRS Research Engineer at the Institute of Biomolecules Max Mousseron (IBMM). After defending his PhD in the field of organic chemistry (2000, University of Bordeaux), he joined the group of Prof. Dave Haddleton at the University of Warwick in UK (Marie-Curie Fellowship, 2000-2002). Then, he moved for a one-year post-doctoral fellow at the University of Bordeaux (LCPO) in the group of Dr. Yves Gnanou (Rhodia funding). In 2004, he joined the IBMM and defended his HDR in 2017. His research interests focus in the field of macromolecular engineering in order to develop original polymeric biomaterials for health applications.
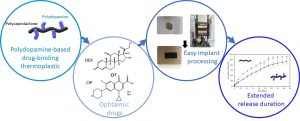
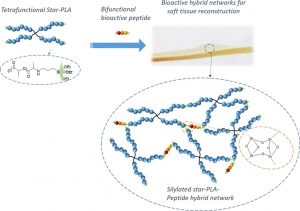
His research interests focus in the field of macromolecular engineering in order to develop original polymeric biomaterials for health applications. Some of his current projects focus on the development of new drug delivery systems, implantable medical devices for bone reconstruction, or bioconjugates for medical diagnosis. He is the co-author of 42 publications and 2 patents in the field of polymer chemistry.
He’s in charge of the “polymer” facility of the SynBio3 platform (IBISA label & ISO9001 certification) dedicated to assist the development of research programs in life science providing biomolecules and polymers of biological and pharmaceutical interest.
Contact:
vincent.darcos(a)umontpellier.fr
(+33) 0411759704
5 recent publications:
El Habnouni, S.; Darcos, V.; Coudane, J., Synthesis and Ring Opening Polymerization of a New Functional Lactone, alpha-Iodo-epsilon-caprolactone: A Novel Route to Functionalized Aliphatic Polyesters. Macromolecular Rapid Communications 2009, 30 (3), 165-169.
Bakkour, Y.; Darcos, V.; Li, S. M.; Coudane, J., Diffusion ordered spectroscopy (DOSY) as a powerful tool for amphiphilic block copolymer characterization and for critical micelle concentration (CMC) determination. Polymer Chemistry 2012, 3 (8), 2006-2010.
Coumes, F.; Huang, C. Y.; Huang, C. H.; Coudane, J.; Domurado, D.; Li, S. M.; Darcos, V.; Huang, M. H., Design and Development of Immunomodulatory Antigen Delivery Systems Based on Peptide/PEG-PLA Conjugate for Tuning Immunity. Biomacromolecules 2015, 16 (11), 3666-3673.
Younis, M.; Darcos, V.; Paniagua, C.; Ronjat, P.; Lemaire, L.; Nottelet, B.; Garric, X.; Bakkour, Y.; El Nakat, J. H.; Coudane, J., MRI-visible polymer based on poly(methyl methacrylate) for imaging applications. Rsc Advances 2016, 6 (7), 5754-5760.
Coumes, F.; Beaute, L.; Domurado, D.; Li, S.; Lecommandoux, S.; Coudane, J.; Darcos, V., Self-assembly of well-defined triblock copolymers based on poly(lactic acid) and poly(oligo(ethylene glycol) methyl ether methacrylate) prepared by ATRP. RSC Advances 2016, 6 (58), 53370-53377