In Vivo Evaluation of the Efficacy and Safety of a Novel Degradable Polymeric Film for the Prevention of Intrauterine Adhesions.
Journal of Minimally Invasive Gynecology (2020)
Stéphanie Huberlant, Salomé Leprince, Lucie Allegre, Sophie Warembourg, Isabelle Leteuff, Hubert Taillades, Xavier Garric., Renaud de Tayrac, Vincent Letouzey.
ABSTRACT
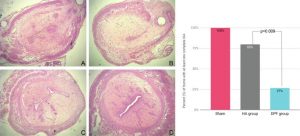
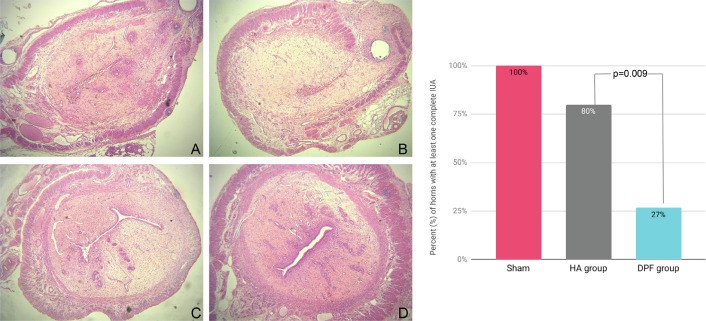
To study the safety of a degradable polymeric film (DPF) and its efficacy on reducing the risk of intrauterine-adhesion (IUA) formation in a rat model.A series of case-control studies relying on random allocation, where feasible.The animal models comprised female and male Oncins France Strain A and female Wistar rats.The Oncins France Strain A rats were used for in vivo evaluation of the impact of the DPF on endometrial thickness and its effect on fertility. For in vivo evaluation of the biologic response, 40 Wistar rats were randomly allocated to intervention and control groups, with matched sampling time after surgery. Finally, for the in vivo evaluation of the DPF’s efficacy on IUA prevention, a total of 24 Wistar rats were divided into 3 groups: 1 treated with the DPF, 1 treated with hyaluronic acid gel, and a sham group. The DPF did not have a significant impact on endometrial thickness, and there were no significant differences in the number of conceived or prematurely terminated pregnancies, confirming its noninferiority to no treatment. The DPF did not induce irritation at 5 days and 28 days. Finally, the DPF significantly reduced the likelihood of complete IUA formation compared with hyaluronic acid gel– and sham-implanted animals, where only 27% of the animals had their uterine cavity obliterated compared with 80% and 100%, respectively.The DPF is a safe film that is effective in preventing IUA formation after intrauterine curettage in rats.