Formulation, physicochemical characterization and stability study of lithium-loaded microemulsion system
Formulation, physicochemical characterization and stability study of lithium-loaded microemulsion system
Int. J. Pharm. 502, 117–124 (2016)
Mouri, A., Legrand, P., El Ghzaoui, A., Dorandeu, C., Maurel, J. C. & Devoisselle, J.-M.
ABSTRACT
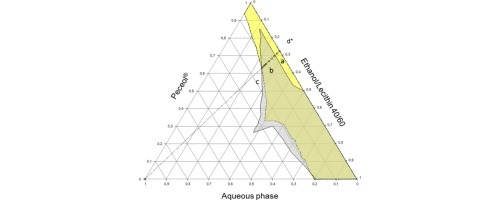
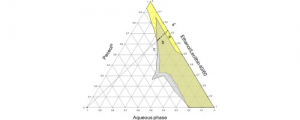
Lithium biocompatible microemulsion based on Peceol®, lecithin, ethanol and water was studied in attempt to identify the optimal compositions in term of drug content, physicochemical properties and stability. Lithium solubilization in microemulsion was found to be compatible with a drug-surfactant binding model. Lithium ions were predominantly solubilized within lecithin head group altering significantly the interfacial properties of the system. Pseudo-ternary phase diagrams of drug free and drug loaded microemulsions were built at constant ethanol/lecithin weight ratio (40/60). Lithium loaded microemulsion has totally disappeared in the Peceol® rich part of phase diagram; critical fractions of lecithin and ethanol were required for the formation of stable microemulsion. The effect of lithium concentration on the properties and physical stability of microemulsions were studied using microscopy, Karl Fischer titrations, rheology analyses, conductivity measurements and centrifugation tests. The investigated microemulsions were found to be stable under accelerated storage conditions. The systems exhibited low viscosity and behaved as Newtonian fluid and no structural transition was shown.


