Coline Pinese

Coline Pinese
Associate professor, Faculty of Pharmacy, University of Montpellier
Coline has been working at the chemistry/biology interface since her doctorate CIFRE obtained in 2014. Her objective was to design hybrid collagen/PLA based copolymer biomaterials for ligament regeneration. She then continued her work at the interface by studying bioactive surfaces functionalized by peptides (antibacterial, osteointegration, wound healing…). Then she became involved into spinal cord regeneration through an association mRNA or siRNA/ nanofibers at Nayang Technological University, Singapore. She finally joined the DBA team as an Associate Professor to develop polymer/peptide hybrid nanomaterials.
Contact:
tel: +33 4 11 75 97 10
coline.pinese@umontpellier.fr
5 recent publications:
- W.Ong, C. Pinese, SY Chew, Scaffold-mediated Sequential Drug/Gene Delivery to Promote Nerve Regeneration and Remyelination following Traumatic Nerve Injuries, Advanced Drug Delivery Reviews (2018) (IF=17.28)
- Pinese, JQ. Lin, U. Milbreta, Y. Wang, KW. Leong, SY Chew, Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater. 76, 164–177 (2018). (IF=6.38)
- Gangolphe, S. Déjean, A. Bethry, S. Hunger, C. Pinese, X. Garric, F. Bossard, B. Nottelet, Degradable multi(aryl-azide) star copolymer as universal photo-crosslinker for elastomeric scaffolds, Materials Today Chemistry 12, (2019) 209-221 (IF=NA)




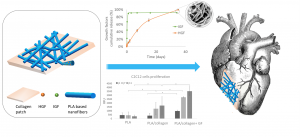
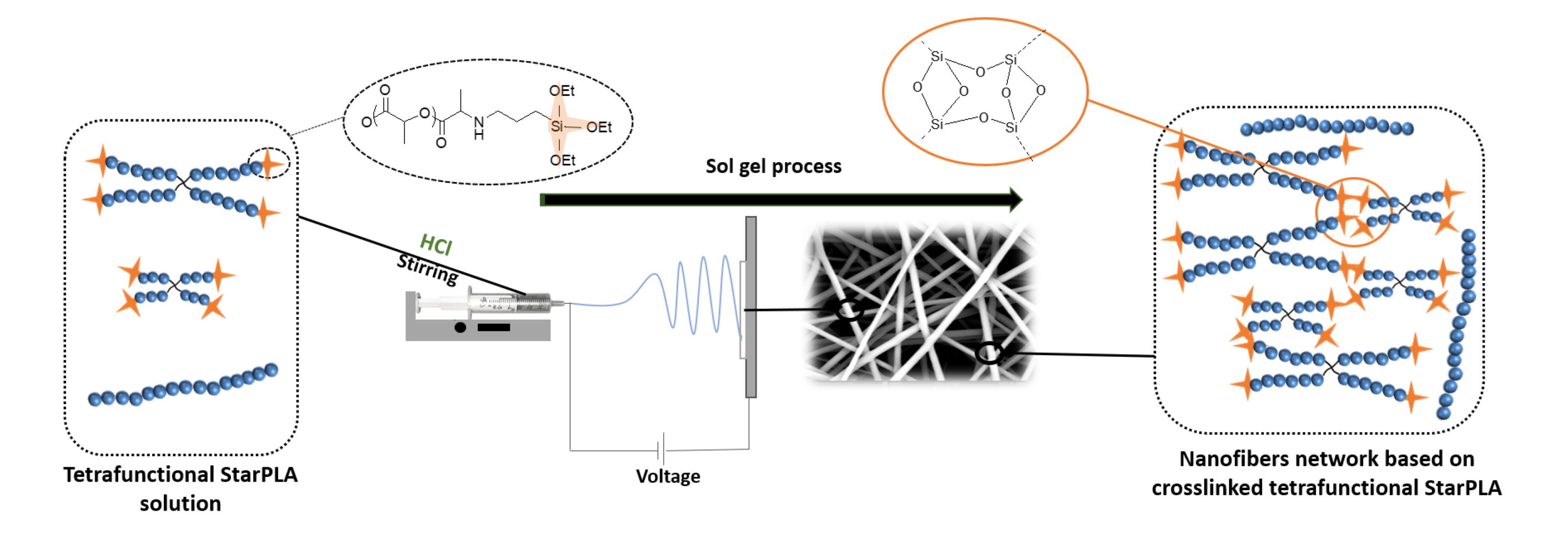
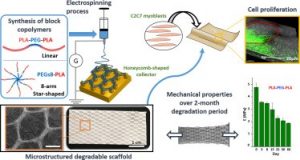 Electrospun scaffolds combine suitable structural characteristics that make them strong candidates for their use in tissue engineering. These features can be tailored to optimize other physiologically relevant attributes (e.g. mechanical anisotropy and cellular affinity) while ensuring adequate degradation rates of the
Electrospun scaffolds combine suitable structural characteristics that make them strong candidates for their use in tissue engineering. These features can be tailored to optimize other physiologically relevant attributes (e.g. mechanical anisotropy and cellular affinity) while ensuring adequate degradation rates of the