Xavier Garric

Xavier Garric
Professor, Faculty of pharmacy, University of Montpellier
Xavier Garric was born in Nice (France) in 1977. He first received his PharmD degree in 2001 before he obtained his PhD in 2004 from the University of Montpellier I, under the supervision of Doctors Michel Vert and Jean-Pierre Molès in the field of degradable polymeric scaffold in skin engineering. He joined the group of Doctor Michel Vert as an Assistant Professor at the University of Montpellier, in 2005. He was appointed Professor of Polymer Chemistry at the Faculty of Pharmacy in 2013 and became team leader in September 2018. His research interests are focused on biomedical applications of polymers and especially for the design of new drug delivery systems and degradable medical devices.
Part of his recent research is related to the design of an anti-adhesion, self-expanding and degradable medical device for the prevention of intra-uterine adhesions. This work led to the creation of the Womed start-up in 2018 of which he is co-founder and scientific advisor.
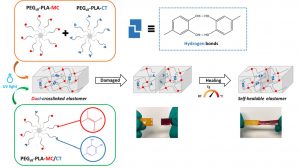
Another part of his research focuses on degradable elastomers for tissue engineering applications as the well as the development of new drug eluting system for implantable medical device.
Xavier is co-author of over 46 papers and 5 patents.
He is co-head of the master’s degree in health engineering, he is deputy director of the scientific chemistry department at the University of Montpellier.
Contact:
xavier.garric(a)umontpellier.fr
+33411759693
5 recent publications:
Pinese C, Gagnieu C, Nottelet B, Rondot-Couzin C, Hunger S, Coudane J, Garric X. In vivo evaluation of hybrid patches composed of PLA based copolymers and collagen/chondroitin sulfate for ligament tissue regeneration. J Biomed Mater Res B 2017;105:1778-88.
Guillaume O, Garric X, Lavigne JP, Van Den Berghe H, Coudane J. Multilayer, degradable coating as a carrier for the sustained release of antibiotics: Preparation and antimicrobial efficacy in vitro. J Control Release 2012;162:492-501. IF 2016 = 7.786
Blanquer S, Guillaume O, Letouzey V, Lemaire L, Franconi F, Paniagua C, Coudane J, Garric X. New magnetic-resonance-imaging-visible poly(epsilon-caprolactone)-based polyester for biomedical applications. Acta Biomater 2012;8:1339-47. IF 2016 = 6.319
Morille M, Tran Van T, Garric X, Coudane J, Venier-Julienne MC, Montero-Menei C. Microsphere compositions, preparation and method and applications thereof. WO2013144341
Coudane J, Leprince S, Garric X, Paniagua C, Huberlant S, Letouzey V. Composition of diblock and triblock copolymers and the use thereof in the prevention of tissues adhesions. WO2016020613