- All
- 2013
- 2014
- 2015
- 2016
- 2017
- 2018
- 2019
- 2020
- 2021
- 2022
- 2023
- A.ElGhzaoui
- B.Nottelet
- C.Pinese
- H.VanDenBerghe
- J.Coudane
- M.Boustta
- M.Vert
- V.Darcos
- X.Garric
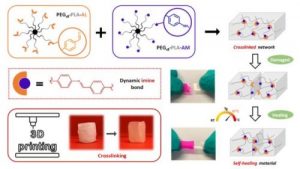
Dynamic and degradable imine-based networks for 3D-printing of soft elastomeric self-healable devices
This is custom heading element
Adv. Mater. Interf. 2300066 (2023)
Mathilde Grosjean, Lucien Guth, Stéphane Déjean, Cédric Paniagua, Benjamin Nottelet
ABSTRACT
Self-healable degradable networks encounter a growing popularity for biomedical applications due to their ability to recover their properties after damage. Self-healable hydrogels dominate with applications in tissue engineering and drug delivery. On the opposite and despite their potential for medical devices, self-healable elastomers remain scarce, especially if they must be compatible with fused deposition modeling (FDM) 3D-printing and self-heal at physiological temperature under hydrated state. These unmet challenges are addressed in this work with degradable elastomeric networks based on dynamic imine bonds prepared from multi(aldehyde) and multi(amine) hydrophobic PEG-PLA star-shaped copolymers. The star topology of these copolymers is the key feature of our strategy as it allows the design of multifunctional high molecular weights pre-polymers that ensure an efficient dynamic chemical crosslinking while guarantying access to the FDM process generally restricted to thermoplastics. The proposed elastomeric networks combine high self-healing efficiencies at 37°C (> 97 %) with mechanical properties compatible with soft tissues and a linear degradation profile. Their FDM processing to produce self-healable tubular devices is demonstrated. Finally, their cytocompatibility is assessed and confirm their potential as biodegradable elastomeric networks to be used for the design of self-healable 3D-printed devices for biomedical applications.
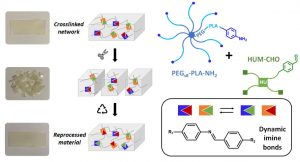
Dynamic PEG−PLA/Hydroxyurethane Networks Based on Imine Bonds as Reprocessable Elastomeric Biomaterials
This is custom heading element
Biomacromolecules 24,3472–3483 (2023)
Mathilde Grosjean, Dimitri Berne, Sylvain Caillol, Vincent Ladmiral, Benjamin Nottelet
ABSTRACT
The development of dynamic covalent chemistry opens the way to the design of materials able to be reprocessed by an internal exchange reaction under thermal stimulus. Imine exchange differs from other exchange reactions by its relatively low temperature of activation. In this study, amine-functionalized star-shaped PEG–PLA and an aldehyde-functionalized hydroxyurethane modifier were combined to produce PEG–PLA/hydroxyurethane networks incorporating imine bonds. The thermal and mechanical properties of these new materials were evaluated as a function of the initial ratio of amine/aldehyde used during synthesis. Rheological analyses highlighted the dynamic behavior of these vitrimers at moderate temperature (60–85 °C) and provided the flow activation energies. Additionally, the reprocessability of these PEG–PLA/hydroxyurethane vitrimers was assessed by comparing the material properties before reshaping and after three reprocessing cycles (1 ton, 1 h, 70 °C). Hence, these materials can easily be designed to satisfy a specific medical application without properties loss. This work opens the way to the development of a new generation of dynamic materials combining degradable PEG–PLA copolymers and hydroxyurethane modifiers, which could find applications in the shape of medical devices on-demand under mild conditions.
Degradable Self-healable Networks for Use in Biomedical Applications
This is custom heading element
Adv. Funct. Mater 2205315 (2023)
Mathilde Grosjean, Louis Gangolphe, Benjamin Nottelet
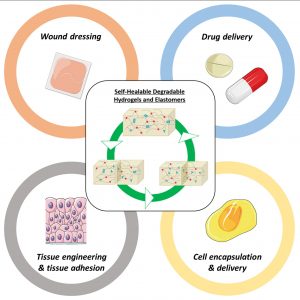
ABSTRACT
Among biomaterials, 3D networks with capacities to absorb and retain large quantities of water (hydrogels) or withstand significant deformation and stress while recovering their initial structures at rest (elastomers) are largely used in biomedical applications. However, when damaged, they cannot recover their initial structures and properties. To overcome this limitation and satisfy the requirements of the biomedical field, self-healable hydrogels and
elastomers designed using (bio)degradable or bioeliminable polymer chains have been developed and are becoming increasingly popular. This review presents the latest advances in the field of self-healing degradable/bioeliminable networks designed for use in health applications. The strategies used to develop such networks based on reversible covalent or physical cross-linking or their combination via dual/multi-cross-linking approaches are analyzed in detail. The key parameters of these hydrogels and elastomers, such as mechanical properties, repair and degradation times, and healing efficiencies, are critically considered in terms of their suitabilities in biomedical applications. Finally, their current and prospective uses as biomaterials in the fields of tissue engineering, drug/cell delivery, and medical devices are presented, followed by the remaining challenges faced to ensure the further success of degradable self-healable networks.
Mechanical Evaluation of Hydrogel–Elastomer Interfaces Generated through Thiol–Ene Coupling
This is custom heading element
ACS Appl. Polym. Mater 5, 1364-1373 (2023)
Khai D. Q. Nguyen, Stéphane Dejean, Benjamin Nottelet, Julien E. Gautrot
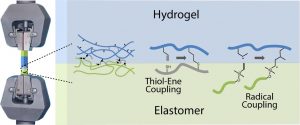
ABSTRACT
The formation of hybrid hydrogel–elastomer scaffolds is an attractive strategy for the formation of tissue engineering constructs and microfabricated platforms for advanced in vitro models. The emergence of thiol–ene coupling, in particular radical-based, for the engineering of cell-instructive hydrogels and the design of elastomers raises the possibility of mechanically integrating these structures without relying on the introduction of additional chemical moieties. However, the bonding of hydrogels (thiol–ene radical or more classic acrylate/methacrylate radical-based) to thiol–ene elastomers and alkene-functional elastomers has not been characterized in detail. In this study, we quantify the tensile mechanical properties of hybrid hydrogel samples formed of two elastomers bonded to a hydrogel material. We examine the impact of radical thiol–ene coupling on the crosslinking of both elastomers (silicone or polyesters) and hydrogels (based on thiol–ene crosslinking or diacrylate chemistry) and on the mechanics and failure behavior of the resulting hybrids. This study demonstrates the strong bonding of thiol–ene hydrogels to alkene-presenting elastomers with a range of chemistries, including silicones and polyesters. Overall, thiol–ene coupling appears as an attractive tool for the generation of strong, mechanically integrated, hybrid structures for a broad range of applications.
Release kinetics of dexamethasone phosphate from porous chitosan: comparison of aerogels and cryogels
This is custom heading element
Biomacromolecules XXX, XXX (2023)
Coraline Chartier, Sytze Buwalda, Blessing C. Ilochonwu, Hélène Van Den Berghe, Audrey Bethry, Tina Vermonden, Martina Viola, Benjamin Nottelet, Tatiana Budtova
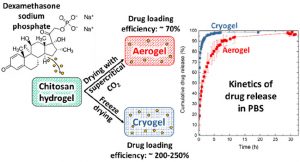
ABSTRACT
Porous chitosan materials as potential wound dressings were prepared via dissolution of chitosan, nonsolvent-induced phase separation in NaOH−water, formation of a hydrogel, and either freeze-drying or supercritical CO2 drying, leading to “cryogels” and “aerogels”, respectively. The hydrophilic drug dexamethasone sodium phosphate was loaded by impregnation of chitosan hydrogel, and the release from cryogel or aerogel was monitored at two pH values relevant for wound healing. The goal was to compare the drug-loading efficiency and release behavior from aerogels and cryogels as a function of the drying method, the materials’ physicochemical properties (density, morphology), and the pH of the release medium. Cryogels exhibited a higher loading efficiency and a faster release in comparison with aerogels. A higher sample density and lower pH value of the release medium resulted in a more sustained release in the case of aerogels. In contrast, for cryogels, the density and pH of the release medium did not noticeably influence release kinetics. The Korsmeyer−Peppas model showed the best fit to describe the release from the porous chitosan materials into the different media.
Development of hybrid bioactive nano fibers composed of star Poly (lactic acid ) and gelatin by sol – gel crosslinking during the electrospinning process
Nanotechnology 34 (2023) 485701
Karima Belabbes, Matthieu Simon, Christopher Yusef Leon-Valdivieso, Mathilde Massonié, Audrey Bethry, Gilles Subra, Xavier Garric and Coline Pinese
ABSTRACT
The design of a biomimetic scaffold is a major challenge in tissue engineering to promote tissue reconstruction. The use of synthetic polymer nano fi bers is widely described as they provide biocompatible matrices whose topography mimics natural extracellular matrix ( ECM ) . To closely match the biochemical composition of the ECM, bioactive molecules such as gelatin are added to the nano fi bers to enhance cell adhesion and proliferation. To overcome the rapid solubilization of gelatin in biological fl uids and to allow a lasting biological effect, the covalent crosslinking of this macromolecule in the network is crucial. The sol – gel route offers the possibility of gentle crosslinking during shaping but is rarely combined with electrospinning. In this study, we present the creation of Poly ( lactic acid )/ Gelatin hybrid nano fi bers by sol – gel route during electrospinning. To enable sol – gel crosslinking, we synthesized star-shaped PLA and functionalized it with silane groups; then we functionalized gelatin with the same groups for their subsequent reaction with the polymer and thus the creation of the hybrid nanonetwork. We evaluated the impact of the presence of gelatin in Poly ( lactic acid )/ Gelatin hybrid nano fi bers at different percentages on the mechanical properties, nanonetwork crosslinking, degradation and biological properties of the hybrid nano fi bers. The addition of gelatin modulated nanonetwork crosslinking that impacted the stiffness of the nano fi bers, resulting in softer materials for the cells. Moreover, these hybrid nano fi bers also showed a signi fi cant improvement in fi broblast proliferation and present a degradation rate suitable for tissue reconstruction. Finally, the bioactive hybrid nano fi bers possess versatile properties, interesting for various potential applications in tissue reconstruction.
Keywords: silylated star PLA, silylated gelatin, hybrid 3D network, bioactive scaffolds, tissue reconstruction
Preliminary in vivo study of biodegradable PLA-PEU-PLA anti-adhesion membranes in a rat Achilles tendon model of peritendinous adhesions
BIOMATERIALS SCIENCE 2022,10, 1776-1786
Hadda, Zebiri, Van Den Berghe Helene, Paunet Tom, Wolf-Mandroux Aurelie, Bethry Audrey, Taillades Hubert, Yohan Jean Noel, Yohan Jean Noël, Nelly Pirot, Botteron Catherine, Chammas Michel, Chammas Pierre-Emmanuel and Garric Xavier
ABSTRACT
Peritendinous adhesions are complications known to occur up to 6 weeks after surgery and cause chronic pain and disability. Anti-adhesion barriers are currently the best option for prevention. In a previous study, we designed two biodegradable membranes, D-PACO1 and D-PACO2, based on new triblock copolymers and conducted in vitro evaluations. The membranes maintained filmogenic integrity, had degradation rates that promoted anti-adhesion and were biocompatible, suggesting their safe and effective use as anti-adhesion devices. To test this hypothesis, we conducted a preliminary in vivo study in a rat model of peritendinous adhesions and evaluated the membranes’ degradation rates, tendon healing and anti-adhesion effect compared to non-surgical and surgical control groups 2 and 10 weeks after surgery. Macroscopic evaluation showed membranes were effective in reducing the extent and severity of adhesions. Membranes acted as physical barriers at 2 weeks and underwent a complete or significant biodegradation at 10 weeks. D-PACO2 had a longer degradation rate compared to D-PACO1, was more effective in reducing adhesions and is expected to be more effective in promoting tendon healing. The tendency of D-PACO1 to promote tendon healing while D-PACO2 did not interfere with healing highlights the need to redesign the porosity of the D-PACO membranes for optimal nutrient diffusion, while maintaining their anti-adhesion effect and clinical usability. Preliminary findings revealed that adhesions form beyond the 6 weeks cited in the literature. In this study, adhesion formation continued for up to 10 weeks, underlining the need to increase the experimental period and sample size of future experiments evaluating anti-adhesion membranes.
Protein-Polymer Bioconjugates Prepared by Post-Polymerization Modification of Alternating Copolymers
EUROPEAN JOURNAL OF ORGANIC CHEMISTRY 2022
Saxer, S.; Erdogan, O.; Paniagua, C.; Chavanieu, A.; Garric, X.; Darcos, V.
ABSTRACT
Protein-polymer bioconjugates have shown great promise in biomedical and life science applications including drug delivery and diagnosis. The current bioconjugation strategies suffer from lack of efficiency and versatility. In this article, poly(styrene-alt-maleic anhydride) copolymers were first prepared by RAFT polymerization and characterized by different analytical techniques. Then, the poly(styrene-alt-maleic anhydride) precursors were functionalized with primary amine such as azidopropylamine and amino poly(ethylene glycol). The reaction of amino compounds with maleic anhydride was found to be a highly efficient, a versatile, and a facile chemical ligation reaction for the synthesis of macromolecules with quantitative yield under mild conditions. The main benefit is the incorporation of a wide range of functionality by easily changing the primary amine compound. For the amphiphilic graft copolymers based on poly(ethylene glycol), aggregation behavior in water was investigated. In a second part, azido-functionalized polystyrene copolymers were used to prepare a new protein-polymer bioconjugate by copper-free click chemistry reaction.
Syntheses of biodegradable graft copolymers from sodium caseinate and poly 3 -caprolactone or poly lactic acid. Applications to the compatibilization of sodium caseinate/polyester blends
Materials Today Chemistry 27 (2023) 101345
L.Viora, T. Tichané, A. Taguet, X. Garric, J. Coudane, H. Van Den Berghe
ABSTRACT
Casein (and its sodium salt, sodium caseinate, SC) is an inexpensive natural milk protein that is used as a biodegradable biomaterial, especially to produce packaging films. However, to enhance some of its properties, it needs to be blended with other polymers, which should preferably be biodegradable such as poly lactic acid (PLA) and poly ε-caprolactone (PCL). New SC-g-PLA and SC-g-PCL graft copolymers have been prepared and unambiguously characterized, in particular by 1H and DOSY NMR. The grafting degrees are high (between 24 and 35% by weight) and result in variations of properties, such as hydrophobicity and thermal properties. The microstructures of SC/PLA and SC/PCL blends were studied and compared, with and without the addition of the SC-g-PLA and SC-g-PCL copolymers to test the compatibilization capacity of these new biodegradable copolymers.
Chemical modification of edible sodium caseinate: A new grafting method of oleic acid. Characterization and thermal properties of the conjugate
Food Chemistry Volume 408, 15 May 2023, 135140
Teddy Tichané, Laurianne Viora, Xavier Garric, Emmanuel Klem-Robin, Jean Coudane, Hélène Van Den Berghe
ABSTRACT
Sodium caseinate is a well-known amphiphilic protein derived from natural products currently used for the preparation of edible films. To improve some properties, especially to decrease the hydrophilicity and water solubility of the caseinate, the covalent grafting of a hydrophobic edible fatty acid, namely oleic acid, onto caseinate, appears to be a solution. We describe a new synthesis method for the chemical modification of sodium caseinate involving the synthesis of an acid chloride derivative from oleic acid and a phase transfer catalysis reaction in a biphasic medium. Under these conditions, free amine and alcohol groups of the caseinate are likely to be grafted with a fairly high (>50 %) substitution degree. The caseinate derivative is finely characterized, in particular by DOSY NMR, to assess the formation of a casein/oleic acid grafted compound as well as the absence of residual oleic acid.
Dual-crosslinked degradable elastomeric networks with self-healing properties: bringing multi(catechol) star block copolymers into play
This is custom heading element
ACS Appl. Mater. Interfaces 15, 2077-2091 (2023)
Mathilde Grosjean, Louis Gangolphe, Stéphane Dejean, Sylvie Hunger, Audrey Bethry, Frédéric Bossard, Xavier Garric, Benjamin Nottelet
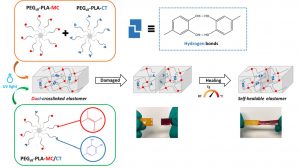
ABSTRACT
In the biomedical field, degradable chemically crosslinked elastomers are interesting materials for tissue engineering applications since they present rubber-like mechanical properties matching with those of soft tissues and are able to preserve their 3D structure over degradation. Their use in biomedical applications requires surgical handling and implantation that can be source of accidental damages responsible for loss of properties. Therefore, their inability to be healed after damage or breaking can be a major drawback. In this work, biodegradable dual-crosslinked networks that exhibit fast and efficient self-healing properties at 37 °C are designed. Self-healable dual-crosslinked (chemically and physically) elastomeric networks are prepared from two methods. The first approach is based on the mix of hydrophobic PEG-PLA star-shaped copolymers functionalized either with catechol or methacrylate moieties. In the second approach, hydrophobic bifunctional PEG-PLA star-shaped copolymers with both catechol and methacrylate on their structure are used. In the two systems the supramolecular network is responsible for the self-healing properties thanks to the dynamic dissociation/re-association of the numerous hydrogen bonds between the catechol groups, whereas the covalent network ensures mechanical properties similar to pure methacrylate networks. The self-healable materials display mechanical properties that are compatible with soft tissues and exhibit linear degradation because of the chemical crosslinks. The performances of networks from mix copolymers vs. bifunctional copolymers are compared and demonstrate the superiority of the later. The biocompatibility of the materials is also demonstrated and confirm the potential of these degradable self-healable elastomeric networks to be used for the design of temporary medical devices.
Degradable Bioadhesives Based on Star PEG–PLA Hydrogels for Soft Tissue Applications
This is custom heading element
Biomacromolecules XX, XX (2022)
Mathilde Grosjean, Edouard Girard, Audrey Bethry, Grégory Chagnon, Xavier Garric, Benjamin Nottelet
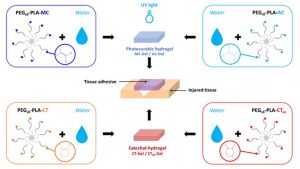
ABSTRACT
Tissue adhesives are interesting materials for wound treatment as they present numerous advantages compared to traditional methods of wound closure such as suturing and stapling. Nowadays, fibrin and cyanoacrylate glues are the most widespread commercial biomedical adhesives, but these systems display some drawbacks. In this study, degradable bioadhesives based on PEG–PLA star-shaped hydrogels are designed. Acrylate, methacrylate, and catechol functional copolymers are synthesized and used to design various bioadhesive hydrogels. Various types of mechanisms responsible for adhesion are investigated (physical entanglement and interlocking, physical interactions, chemical bonds), and the adhesive properties of the different systems are first studied on a gelatin model and compared to fibrin and cyanoacrylate references. Hydrogels based on acrylate and methacrylate reached adhesion strength close to cyanoacrylate (332 kPa) with values of 343 and 293 kPa, respectively, whereas catechol systems displayed higher values (11 and 19 kPa) compared to fibrin glue (7 kPa). Bioadhesives were then tested on mouse skin and human cadaveric colonic tissue. The results on mouse skin confirmed the potential of acrylate and methacrylate gels with adhesion strength close to commercial glues (15–30 kPa), whereas none of the systems led to high levels of adhesion on the colon. These data confirm that we designed a family of degradable bioadhesives with adhesion strength in the range of commercial glues. The low level of cytotoxicity of these materials is also demonstrated and confirm the potential of these hydrogels to be used as surgical adhesives.
Poly(ε-caprolactone)-Based Graft Copolymers: Synthesis Methods and Applications in the Biomedical Field: A Review
This is custom heading element
Jean Coudane, Benjamin Nottelet, Julia Mouton, Xavier Garric, Hélène Van Den Berghe
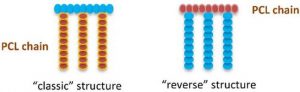
ABSTRACT
Synthetic biopolymers are attractive alternatives to biobased polymers, especially because they rarely induce an immune response in a living organism. Poly ε-caprolactone (PCL) is a well-known synthetic aliphatic polyester universally used for many applications, including biomedical and environmental ones. Unlike poly lactic acid (PLA), PCL has no chiral atoms, and it is impossible to play with the stereochemistry to modify its properties. To expand the range of applications for PCL, researchers have investigated the possibility of grafting polymer chains onto the PCL backbone. As the PCL backbone is not functionalized, it must be first functionalized in order to be able to graft reactive groups onto the PCL chain. These reactive groups will then allow the grafting of new reagents and especially new polymer chains. Grafting of polymer chains is mainly carried out by “grafting from” or “grafting onto” methods. In this review we describe the main structures of the graft copolymers produced, their different synthesis methods, and their main characteristics and applications, mainly in the biomedical field.
Bioresorbable bilayered elastomers/hydrogels constructs with gradual interfaces for the fast actuation of self-rolling tubes
This is custom heading element
ACS Appl. Mater. Interfaces 14, 43719–43731 (2022)
Mathilde Grosjean, Sidzigui Ouedraogo, Stéphane Déjean, Xavier Garric, Valeriy Luchnikov, Arnaud Ponche, Noëlle Mathieu, Karine Anselme, Benjamin Nottelet
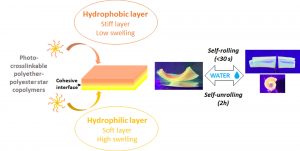
ABSTRACT
In the biomedical field, self-rolling materials provide interesting opportunities to develop medical devices suitable for drug or cell encapsulation. However, to date a major limitation for medical applications is the use of non-biodegradable and non-biocompatible polymers that are often reported for such applications, or the slow actuation witnessed with degradable systems. In this work, biodegradable self-rolling tubes that exhibit a spontaneous and rapid actuation when immersed in water are designed. Photo-crosslinkable hydrophilic and hydrophobic PEG-PLA star-shaped copolymers are prepared and used to prepare bilayered constructs. Thanks to the discrete mechanical and swelling properties of each layer and the cohesive/gradual nature of the interface, the resulting bilayered films are able to self-roll in water in less than 30 seconds depending on the nature of the hydrophilic layer and on the shape of the sample. The cytocompatibility and degradability of the materials are demonstrated and confirm the potential of such self-rolling resorbable biomaterials in the field of temporary medical devices.
Polyester-polydopamine copolymers for intravitreal drug delivery: role of polydopamine drug-binding properties on extending drug release
This is custom heading element
Biomacromolecules 23, 4388-4400, (2022)
Floriane Bahuon, Vincent Darcos, Sulabh Patel, Zana Marin, Jean Coudane, Grégoire Schwach, and Benjamin Nottelet
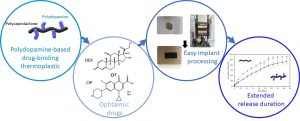
ABSTRACT
This work reports on a novel polyester copolymer containing poly(dopamine), a synthetic analogue of natural melanin, evaluated in sustained-release drug delivery system for ocular intravitreal administration of drugs. More specifically, a graft copolymer of poly(ε-caprolactone)-graft-poly(dopamine) (PCL-g-PDA) has been synthesized, and was shown to further extend the drug release benefits of state-of-the-art biodegradable intravitreal implants made of poly(lactide) and poly(lactide-co-glycolide). The innovative biomaterial combines the documented drug-binding properties of melanin naturally present in the eye, with the established ocular tolerability and biodegradation of polyester implants. The PCL-g-PDA copolymer was obtained by a two-step modification of PCL with a final PDA content around 2-3 wt.%, and was fully characterised by SEC, NMR, and DOSY NMR. The thermoplastic nature of PCL-g-PDA allowed its simple processing by hot-melt compression moulding to prepare small implants. The properties of unmodified PCL and PCL-g-PDA implants were studied and compared in terms of thermal properties (DSC), thermal stability (TGA), degradability and in vitro cytotoxicity. PCL and PCL-g-PDA implants exhibited similar degradation properties in vitro and were both stable under physiological conditions over 110 days. Likewise, both materials were non-cytotoxic towards L929 and ARPE-19 cells. The drug-loading and in vitro release properties of the new materials were investigated with dexamethasone (DEX) and ciprofloxacin hydrochloride (CIP) as representative drugs featuring low and high melanin binding affinities, respectively. In comparison to unmodified PCL, PCL-g-PDA implants showed significant extension of drug release most likely because of specific drug-catechol interaction with the PDA moieties of the copolymer. The present study confirms the advantages of designing PDA-containing polyesters as a class of biodegradable and biocompatible thermoplastics that can modulate and remarkably extend drug release kinetics thanks to their unique drug binding properties, especially, but not limited to, for ocular applications.
Design of Hybrid Polymer Nanofiber/Collagen Patches Releasing IGF and HGF to Promote Cardiac Regeneration
Eloise Kerignard, Audrey Bethry, Chloé Falcoz, Benjamin Nottelet and Coline Pinese
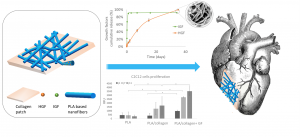
ABSTRACT
Cardiovascular diseases are the leading cause of death globally. Myocardial infarction in particular leads to a high rate of mortality, and in the case of survival, to a loss of myocardial functionality due to post-infarction necrosis. This functionality can be restored by cell therapy or biomaterial implantation, and the need for a rapid regeneration has led to the development of bioactive patches, in particular through the incorporation of growth factors (GF). In this work, we designed hybrid patches composed of polymer nanofibers loaded with HGF and IGF and associated with a collagen membrane. Among the different copolymers studied, the polymers and their porogens PLA-Pluronic-PLA + PEG and PCL + Pluronic were selected to encapsulate HGF and IGF. While 89 and 92% of IGF were released in 2 days, HGF was released up to 58% and 50% in 35 days from PLA-Pluronic-PLA + PEG and PCL + Pluronic nanofibers, respectively. We also compared two ways of association for the loaded nanofibers and the collagen membrane, namely a direct deposition of the nanofibers on a moisturized collagen membrane (wet association), or entrapment between collagen layers (sandwich association). The interfacial cohesion and the degradation properties of the patches were evaluated. We also show that the sandwich association decreases the burst release of HGF while increasing the release efficiency. Finally, we show that the patches are cytocompatible and that the presence of collagen and IGF promotes the proliferation of C2C12 myoblast cells for 11 days. Taken together, these results show that these hybrid patches are of interest for cardiac muscle regeneration.
Creation of a Stable Nanofibrillar Scaffold Composed of Star-Shaped PLA Network Using Sol-Gel Process during Electrospinning
Karima Belabbes, Coline Pinese *, Christopher Yusef Leon-Valdivieso, Audrey Bethry, Xavier Garric
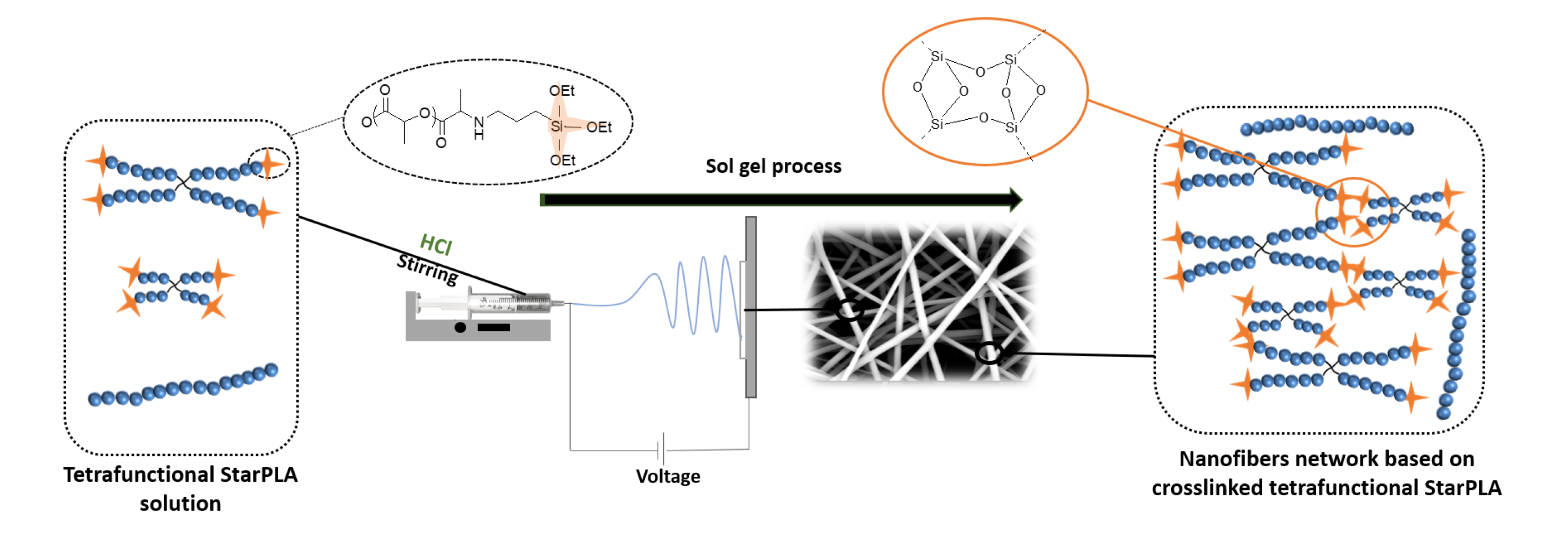
ABSTRACT
PLA nanofibers are of great interest in tissue engineering due to their biocompatibility and morphology; moreover, their physical properties can be tailored for long-lasting applications. One of the common and efficient methods to improve polymer properties and slow down their degradation is sol-gel covalent crosslinking. However, this method usually results in the formation of gels or films, which undervalues the advantages of nanofibers. Here, we describe a dual process sol-gel/electrospinning to improve the mechanical properties and stabilize the degradation of PLA scaffolds. For this purpose, we synthesized star-shaped PLAs and functionalized them with triethoxysilylpropyl groups (StarPLA-PTES) to covalently react during nanofibers formation. To achieve this, we evaluated the use of (1) a polymer diluent and (2) different molecular weights of StarPLA on electrospinnability, StarPLA-PTES condensation time and crosslinking efficiency. Our results show that the diluent allowed the fiber formation and reduced the condensation time, while the addition of low-molecular-weight StarPLA-PTES improved the crosslinking degree, resulting in stable matrices even after 6 months of degradation. Additionally, these materials showed biocompatibility and allowed the proliferation of fibroblasts. Overall, these results open the door to the fabrication of scaffolds with enhanced stability and prospective long-term applications
Peptide-guided self-assembly of polyethylene glycol-b-poly(ε-caprolactone-g-peptide) block copolymers
This is custom heading element
Eur. Pol. J. 176, 111386 (2022)
Ayman El Jundi, Matthias Mayor, Enrique Folgado, Chaimaa Gomri, Belkacem Tarek Benkhaled, Arnaud Chaix, Pascal Verdie, Benjamin Nottelet, Mona Semsarilar

ABSTRACT
Biodegradable poly(ethylene glycol)-b-poly(ε-caprolactone-g-peptide) (PEG-b-PCL-g-peptide) copolymers were synthesized using a combination of ring opening polymerization and thiol-yne photoaddition of peptides on the alkyne functional PCL block. The peptides Phe-Phe, Tyr-Tyr and Arg-Gly-Asp were selected based on the expected interactions (Pi-stacking, H-bonding, electrostatic). The self-assembly of these copolymers was studied via testing the effect of various parameters such as the nature of the solvent and non-solvant as well as their ratio,mixing method, temperature and concentration. Structures obtained by varying these parameters were characterised using transmission electron microscopy (TEM) and dynamic light scattering (DLS). Spherical and lamellar structures (oval leaf-shaped) of different sizes were identified as a function of the conditions. The role of the crystallisation and of the peptides was highlighted with more defined and stable structures obtained for Tyr-Tyr functional copolymers.
Poly(Lactic Acid)-Based Graft Copolymers: Syntheses Strategies and Improvement of Properties for Biomedical and Environmentally Friendly Applications – A Review
This is custom heading element
Jean Coudane , Hélène Van Den Berghe, Julia Mouton, Xavier Garric, Benjamin Nottelet
ABSTRACT
As a potential replacement for petroleum-based plastics, biodegradable bio-based polymers such as poly(lactic acid) (PLA) have received much attention in recent years. PLA is a biodegradable polymer with major applications in packaging and medicine. Unfortunately, PLA is less flexible and has less impact resistance than petroleum-based plastics. To improve the mechanical properties of PLA, PLA-based blends are very often used, but the outcome does not meet expectations because of the non-compatibility of the polymer blends. From a chemical point of view, the use of graft copolymers as a compatibilizer with a PLA backbone bearing side chains is an interesting option for improving the compatibility of these blends, which remains challenging. This review article reports on the various graft copolymers based on a PLA backbone and their syntheses following two chemical strategies: the synthesis and polymerization of modified lactide or direct chemical post-polymerization modification of PLA. The main applications of these PLA graft copolymers in the environmental and biomedical fields are presented.
Tuning the properties of porous chitosan: Aerogels and cryogels
This is custom heading element
Int. J. Biol. Macromol. 202, 215–223 (2022)
Coraline Chartier, Sytze Buwalda, Hélène Van Den Berghe, Benjamin Nottelet, Tatiana Budtova
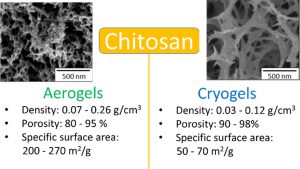
ABSTRACT
Highly porous chitosan-based materials were prepared via dissolution, non-solvent induced phase separation and drying using different methods. The goal was to tune the morphology and properties of chitosan porous materials by varying process parameters. Chitosan concentration, concentration of sodium hydroxide in the coagulation bath and aging time were varied. Drying was performed via freeze-drying leading to “cryogels” or via drying with supercritical CO2 leading to “aerogels”. Cryogels were of lower density than aerogels (0.03–0.12 g/cm3 vs 0.07–0.26 g/cm3, respectively) and had a lower specific surface area (50–70 vs 200–270 m2/g, respectively). The absorption of simulated wound exudate by chitosan aerogels and cryogels was studied in view of their potential applications as wound dressing. Higher absorption was obtained for cryogels (530–1500%) as compared to aerogels (200–610%).
Electrospun microstructured PLA-based scaffolds featuring relevant anisotropic, mechanical and degradation characteristics for soft tissue engineering
Materials Science and Engineering: C Volume 129, October 2021, 112339
Louis Gangolphe, Christopher Y.Leon Valdivieso, Benjamin Nottelet, Stéphane Déjean, Audrey Bethry, Coline Pinese, Frédéric Bossard and Xavier Garric
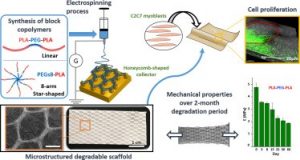 Electrospun scaffolds combine suitable structural characteristics that make them strong candidates for their use in tissue engineering. These features can be tailored to optimize other physiologically relevant attributes (e.g. mechanical anisotropy and cellular affinity) while ensuring adequate degradation rates of the biomaterial. Here, we present the fabrication of microstructured scaffolds by using a combination of micropatterned electrospinning collectors (honeycomb- or square-patterned) and poly(lactic acid) (PLA)-based copolymers (linear or star-shaped). The resulting materials showed appropriate macropore size and fiber alignment that were key parameters to enhance their anisotropic properties in protraction. Moreover, their elastic modulus, which was initially similar to that of soft tissues, gradually changed in hydrolytic conditions, matching the degradation profile in a 2- to 3-month period. Finally, honeycomb-structured scaffolds exhibited enhanced cellular proliferation compared to standard electrospun mats, while cell colonization was shown to be guided by the macropore contour. Taking together, these results provide new insight into the rational design of microstructured materials that can mimic the progressive evolution of properties in soft tissue regeneration
Electrospun scaffolds combine suitable structural characteristics that make them strong candidates for their use in tissue engineering. These features can be tailored to optimize other physiologically relevant attributes (e.g. mechanical anisotropy and cellular affinity) while ensuring adequate degradation rates of the biomaterial. Here, we present the fabrication of microstructured scaffolds by using a combination of micropatterned electrospinning collectors (honeycomb- or square-patterned) and poly(lactic acid) (PLA)-based copolymers (linear or star-shaped). The resulting materials showed appropriate macropore size and fiber alignment that were key parameters to enhance their anisotropic properties in protraction. Moreover, their elastic modulus, which was initially similar to that of soft tissues, gradually changed in hydrolytic conditions, matching the degradation profile in a 2- to 3-month period. Finally, honeycomb-structured scaffolds exhibited enhanced cellular proliferation compared to standard electrospun mats, while cell colonization was shown to be guided by the macropore contour. Taking together, these results provide new insight into the rational design of microstructured materials that can mimic the progressive evolution of properties in soft tissue regeneration
Star-poly(lactide)-peptide hybrid networks as bioactive materials
Eur. Pol. J. 139, 109990 (2020)
L.V. Arsenie, C. Pinese, A. Bethry, L. Valot, P. Verdie, B. Nottelet, G. Subra, V. Darcos, X. Garric
ABSTRACT
Abstract Poly(lactide) (PLA) is a widely used biomaterial in many biomedical applications. However, it is inert and therefore lacks bioactivity, which is a major drawback in addressing tissue regeneration issues. This work aims to develop new implantable biomaterials composed of PLAs functionalized with bioactive peptides. For that purpose, we set up an original synthesis based on star-PLA bearing triethoxysilyl propyl groups (PLA-PTES) and bifunctional silylated peptides that react together via sol-gel process to create a bioactive network. We demonstrate that the molecular weight of the PLA and the quantity of peptide have a large influence on the crosslinking efficiency, the mechanical properties and the biodegradability of the resulting materials. The presence of peptide increases the crosslinking efficiency of the networks resulting in more rigid networks with stable mechanical properties up to 8 weeks. At last, the potential of this new type of hybrid biomaterials for soft tissue engineering was demonstrated through cells adhesion assays that showed a significant enhancement of fibroblasts adhesion
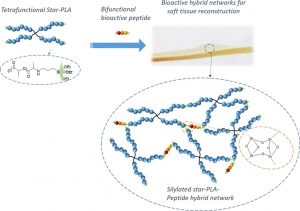
Star-poly(lactide)-peptide hybrid networks as bioactive materials
Long-term in vivo performances of polylactide / iron oxide nanoparticles core-shell fibrous nanocomposites as MRI-visible magneto-scaffolds
This is custom heading element
Biomat. Sci. 9, 6203–6213 (2021)
Awada H., Seene S., Laurencin D., Lemaire L., Franconi F., Bernex F., Bethry A., Garric X., Guari Y., Nottelet B.
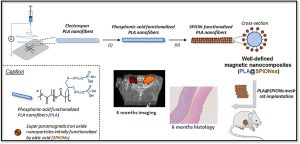
ABSTRACT
There is a growing interest in magnetic nanocomposites in biomaterials science. In particular, nanocomposites that combine poly(lactide) (PLA) nanofibers and super paramagnetic iron oxide nanoparticles (SPIONs), which can be obtained by either electrospinning of a SPIONs suspension in PLA or by precipitating SPIONs at the surface of PLA, are well documented in the literature. However, these two classical processes yield nanocomposites with altered materials properties, and their long-term in vivo fate and performances have in most cases only been evaluated over short periods of time. Recently, we reported a new strategy to prepare well-defined PLA@SPIONs nanofibers with a quasi-monolayer of SPIONs anchored at the surface of PLA electrospun fibers. Herein, we report on a 6-month in vivo rat implantation study with the aim of evaluating the long-term magnetic resonance imaging (MRI) properties of this new class of magnetic nanocomposites, as well as their tissue integration and degradation. Using clinically relevant T2-weighted MRI conditions, we show that the PLA@SPIONs nanocomposites are clearly visible up to 6 months. We also evaluate here by histological analyses the slow degradation of the PLA@SPIONs, as well as their biocompatibility. Overall, these results make these nanocomposites attractive for the development of magnetic biomaterials for biomedical applications.
Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro
This is custom heading element
Acta Biomaterialia 131, 302–313 (2021)
Funnell J.L., Ziemba A.M., Nowak J.F., Awada H., Prokopiou N., Samuel J., Guari Y., Nottelet B., Gilbert R.J.
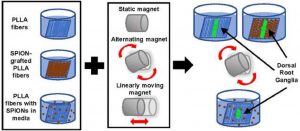
ABSTRACT
Magnetic fiber composites combining superparamagnetic iron oxide nanoparticles (SPIONs) and electrospun fibers have shown promise in tissue engineering fields. Controlled grafting of SPIONs to the fibers post-electrospinning generates biocompatible magnetic composites without altering desired fiber morphology. Here, for the first time, we assess the potential of SPION-grafted scaffolds combined with magnetic fields to promote neurite outgrowth by providing contact guidance from the aligned fibers and mechanical stimulation from the SPIONs in the magnetic field. Neurite outgrowth from primary rat dorsal root ganglia (DRG) was assessed from explants cultured on aligned control and SPION-grafted electrospun fibers as well as on non-grafted fibers with SPIONs dispersed in the culture media. To determine the optimal magnetic field stimulation to promote neurite outgrowth, we generated a static, alternating, and linearly moving magnet and simulated the magnetic flux density at different areas of the scaffold over time. The alternating magnetic field increased neurite length by 40% on control fibers compared to a static magnetic field. Additionally, stimulation with an alternating magnetic field resulted in a 30% increase in neurite length and 62% increase in neurite area on SPION-grafted fibers compared to DRG cultured on PLLA fibers with untethered SPIONs added to the culture media. These findings demonstrate that SPION-grafted fiber composites in combination with magnetic fields are more beneficial for stimulating neurite outgrowth on electrospun fibers than dispersed SPIONs.
Well-defined polyester-grafted silica nanoparticles for biomedical applications: Synthesis and quantitative characterization
Lagarrigue P., Soulié J., Grossin D., Dupret-Bories A., Combes C., Darcos V.
ABSTRACT
Polyester-based composites with silica nanoparticles fillers are promising candidates as biomaterials due to improved mechanical and biological properties. However, nanofillers use generally leads to an inhomogeneous distribution inside the polymer matrix because of agglomeration, decreasing composites overall performances. To improve nanofillers dispersion, the aim of this study is to prepare and characterize poly(D,L lactide) grafted silica nanoparticles using “grafting to” method and to quantify the amount of grafted poly(D,L lactide). Firstly, well-defined N hydroxysuccinimide ester poly(D,L lactide)s were synthetized through a new pathway. Then, amino-functionalized silica nanoparticles were grafted with those customized polyesters yielding an amide covalent bond between both reagents. Such PDLLA grafted nanoparticles were precisely characterized and the grafting amount was quantified using a dual approach based on TGA and FTIR analysis. The synthesis and the characterization methods developed constitute a robust and reproducible way to design well-defined polymer grafted silica nanoparticles that could be used as nanofillers in polymer matrix nanocomposites for biomedical

Modulation of protein release from penta-block copolymer microspheres European Journal of Pharmaceutics and Biopharmaceutics 152, 175–182 (2020)
European Journal of Pharmaceutics and Biopharmaceutics 152, 175–182 (2020).
Minh-Quan Le, Jean-Christophe Gimel, Xavier Garric, Thao-Quyen Nguyen-Pham, Cédric Paniagua, Jérémie Riou, Marie-Claire Venier-Julienne,
ABSTRACT
Releasing a protein according to a zero-order profile without protein denaturation during the polymeric microparticle degradation process is very challenging. The aim of the current study was to develop protein-loaded microspheres with new PLGA based penta-block copolymers for a linear sustained protein release. Lysozyme was chosen as model protein and 40 µm microspheres were prepared using the solid-in-oil-in-water solvent extraction/evaporation process. Two types of PLGA-P188-PLGA penta-block copolymers were synthetized with two PLGA-segments molecular weight (20 kDa or 40 kDa). The resulting microspheres (50P20-MS and 50P40-MS) had the same size, an encapsulation efficiency around 50–60% but different porosities. Their protein release profiles were complementary: linear but non complete for 50P40-MS, non linear but complete for 50P20-MS. Two strategies, polymer blending and microsphere mixing, were considered to match the release to the desired profile. The (1:1) microsphere mixture was successful. It induced a bi-phasic release with a moderate initial burst (around 13%) followed by a nearly complete linear release for 8 weeks. This study highlighted the potential of this penta-block polymer where the PEO block mass ratio influence clearly the Tg and consequently the microsphere structure and the release behavior at 37 °C. The (1:1) mixture was a starting point but could be finely tuned to control the protein release.
In Vivo Evaluation of the Efficacy and Safety of a Novel Degradable Polymeric Film for the Prevention of Intrauterine Adhesions.
Journal of Minimally Invasive Gynecology (2020)
Stéphanie Huberlant, Salomé Leprince, Lucie Allegre, Sophie Warembourg, Isabelle Leteuff, Hubert Taillades, Xavier Garric., Renaud de Tayrac, Vincent Letouzey.
ABSTRACT
To study the safety of a degradable polymeric film (DPF) and its efficacy on reducing the risk of intrauterine-adhesion (IUA) formation in a rat model.A series of case-control studies relying on random allocation, where feasible.The animal models comprised female and male Oncins France Strain A and female Wistar rats.The Oncins France Strain A rats were used for in vivo evaluation of the impact of the DPF on endometrial thickness and its effect on fertility. For in vivo evaluation of the biologic response, 40 Wistar rats were randomly allocated to intervention and control groups, with matched sampling time after surgery. Finally, for the in vivo evaluation of the DPF’s efficacy on IUA prevention, a total of 24 Wistar rats were divided into 3 groups: 1 treated with the DPF, 1 treated with hyaluronic acid gel, and a sham group. The DPF did not have a significant impact on endometrial thickness, and there were no significant differences in the number of conceived or prematurely terminated pregnancies, confirming its noninferiority to no treatment. The DPF did not induce irritation at 5 days and 28 days. Finally, the DPF significantly reduced the likelihood of complete IUA formation compared with hyaluronic acid gel– and sham-implanted animals, where only 27% of the animals had their uterine cavity obliterated compared with 80% and 100%, respectively.The DPF is a safe film that is effective in preventing IUA formation after intrauterine curettage in rats.
Hyaluronic Acid-Poly(N-acryloyl glycinamide) Copolymers as Sources of Degradable Thermoresponsive Hydrogels for Therapy
Gels 2020, 6(4), 42
Mahfoud Boustta and Michel Vert
ABSTRACT
One-pot free-radical polymerization of N-acryloyl glycinamide in the presence of hyaluronic acid as transfer-termination agent led to new copolymers in high yields without any chemical activation of hyaluronic acid before. All the copolymers formed thermoresponsive hydrogels of the Upper Critical Solution Temperature-type in aqueous media. Gel properties and the temperature of the reversible gel ↔ sol transition depended on feed composition and copolymer concentration. Comparison with mixtures of hyaluronic acid-poly(N-acryloyl glycinamide) failed in showing the expected formation of graft copolymers conclusively because poly(N-acryloyl glycinamide) homopolymers are also thermoresponsive. Grafting and formation of comb-like copolymers were proved after degradation of inter-graft hyaluronic acid segments by hyaluronidase. Enzymatic degradation yielded poly(N-acryloyl glycinamide) with sugar residues end groups as shown by NMR. In agreement with the radical transfer mechanism, the molar mass of these released poly(N-acryloyl glycinamide) grafts depended on the feed composition. The higher the proportion of hyaluronic acid in the feed, the lower the molar mass of poly(N-acryloyl glycinamide) grafts was. Whether molar mass can be made low enough to allow kidney filtration remains to be proved in vivo. Last but not least, Prednisolone was used as model drug to show the ability of the new enzymatically degradable hydrogels to sustain progressive delivery for rather long periods of time in vitro.DPF is a safe film that is effective in preventing IUA formation after intrauterine curettage in rats.
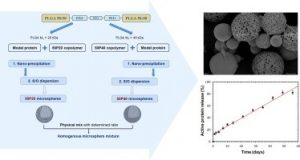
Poly(Aspartic Acid) Functionalized Poly(e-Caprolactone) Microspheres with Enhanced Hydroxyapatite Affinity as Bone Targeting Antibiotic Carriers
This is custom heading element
Rotman S.G., Moriarty T.F., Nottelet B., Grijpma D.W., Eglin D., Guillaume O.
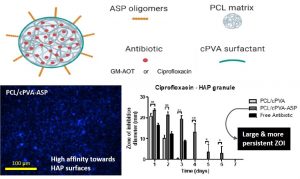
ABSTRACT
Bone infection is a feared complication for patients with surgically fixed bone fractures and local antibiotic delivery is important in prophylaxis and treatment of these infections. Recent studies indicated that Staphylococcus aureus can penetrate bone tissue through micron-sized canaliculi and evade systemic and currently available local antibiotic treatments. Targeting bacteria within the bone requires highly efficient delivery of antimicrobials to the infected bone tissue. In this work, a biodegradable microsphere carrier loaded with antibiotics and with specific affinity to bone mineral was developed. Two widely used antibiotics, i.e. Gentamicin-AOT (GM-AOT) and Ciprofloxacin (CF) were embedded in poly(ϵ-caprolactone) (PCL) microspheres fabricated by oil-in-water emulsion techniques with carboxylated poly(vinyl alcohol) (cPVA) as surfactant. The carboxylic acid groups present at the PCL/cPVA microsphere surface were functionalized with aspartic acid oligomers (ASP) granting bone targeting properties. We report on cPVA synthesis, microsphere formulation and antibiotic loading of PCL/cPVA-ASP microspheres. Antibiotic loaded PCL/cPVA-ASP microspheres show sustained release of its antibiotic load and can inhibit bacterial growth in vitro for up to 6 days. PCL/cPVA-ASP microspheres show enhanced affinity to mineralized substrates compared to non-functionalized PCL/cPVA microspheres. These findings support further development of these bone targeting antibiotic carriers for potential treatment of persistent bone infections.
Evaluation of a biodegradable PLA–PEG–PLA internal biliary stent for liver transplantation: in vitro degradation and mechanical properties
This is custom heading element
J. Biomed. Mater. Res. 1-10, (2020)
Girard E., Chagnon G., Moreau-Gaudry A., Letoublon C., Favier D., Dejean S., Trilling B., Nottelet B.
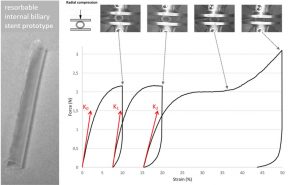
ABSTRACT
Internal biliary stenting during biliary reconstruction in liver transplantation decrease anastomotic biliary complications. Implantation of a resorbable internal biliary stent (RIBS) is interesting since it would avoid an ablation gesture. The objective of present work was to evaluate adequacy of selected PLA-b-PEG-b-PLA copolymers for RIBS aimed to secure biliary anastomose during healing and prevent complications, such as bile leak and stricture. The kinetics of degradation and mechanical properties of a RIBS prototype were evaluated with respect to the main bile duct stenting requirements in liver transplantation. For this purpose, RIBS degradation under biliary mimicking solution versus standard phosphate buffer control solution was discussed. Morphological changes, mass loss, water uptake, molecular weight, permeability, pH variations, and mechanical properties were examined over time. The permeability and mechanical properties were evaluated under simulated biliary conditions to explore the usefulness of a PLA-b-PEG-b-PLA RIBS to secure biliary anastomosis. Results showed no pH influence on the kinetics of degradation, with degradable RIBS remaining impermeable for at least 8 weeks, and keeping its mechanical properties for 10 weeks. Complete degradation is reached at 6 months. PLA-b-PEG-b-PLA RIBS have the required in vitro degradation characteristics to secure biliary anastomosis in liver transplantation and envision in vivo applications
Double hydrophilic block copolymers self-assemblies in biomedical applications
This is custom heading element
Adv. Colloid Interface Sci 283, 102213, (2020)
A. El Jundi, S. Buwalda, Y. Bakkour, X. Garric, B. Nottelet
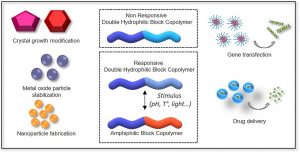
ABSTRACT
Double-hydrophilic block copolymers (DHBCs), consisting of at least two different water-soluble blocks, are an alternative to the classical amphiphilic block copolymers and have gained increasing attention in the field of biomedical applications. Although the chemical nature of the two blocks can be diverse, most classical DHBCs consist of a bioeliminable non-ionic block to promote solubilization in water, like poly(ethylene glycol), and a second block that is more generally a pH-responsive block capable of interacting with another ionic polymer or substrate. This second block is generally non-degradable and the presence of side chain functional groups raises the question of its fate and toxicity, which is a limitation in the frame of biomedical applications. In this review, following a first part dedicated to recent examples of non-degradable DHBCs, we focus on the DHBCs that combine a biocompatible and bioeliminable non-ionic block with a degradable functional block including polysaccharides, polypeptides, polyesters and other miscellaneous polymers. Their use to design efficient drug delivery systems for various biomedical applications through stimuli-dependent self-assembly is discussed along with the current challenges and future perspectives for this class of copolymers.
Degradable double hydrophilic block copolymers and tripartite polyionic complex micelles thereof for small interfering ribonucleic acids (siRNA) delivery
This is custom heading element
J. Colloid Interface 580, 449, (2020)
A. El Jundi, M. Morille, N. Bettache, A. Bethry, J. Berthelot, J. Salvador, S. Hunger, Y. Bakkour, E. Belamie, B. Nottelet
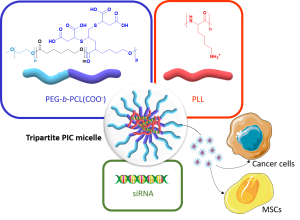
ABSTRACT
Polymer vectors for gene therapy have been largely investigated as an alternative to viral vectors. In particular, double hydrophilic block copolymers (DHBCs) have shown potential in this domain, but to date studies mainly focus on non-degradable copolymers, which may be a restriction for further development. To overcome this limitation, we synthesized a DHBC (PEG43-b-PCL12(COOH)6.5) composed of a poly(ethylene glycol) (PEG) non-ionic and bioeliminable block and a degradable carboxylic acid-functionalized poly(e-caprolactone) (PCL) block. The potential of this DHBC as an original vector for small interfering ribonucleic acids (siRNA) to formulate tripartite polyionic complex (PIC) micelles with poly(lysine) (PLL) was evaluated. We first studied the impact of the charge ratio (R) on the size and the zeta potential of the resulting micelles. With a charge ratio R=1, one formulation with optimized physico-chemical properties showed the ability to complex 75 % of siRNA. We showed a stability of the micelles at pH 7.4 and a disruption at pH 5, which allowed a pH-triggered siRNA release and proved the pH-stimuli responsive character of the tripartite micelles. In addition, the tripartite PIC micelles were shown to be non-cytotoxic below 40 µg/mL. The potential of these siRNA vectors was further evaluated in vitro: it was found that the tripartite PIC micelles allowed siRNA internalization to be 3 times higher than PLL polyplexes in murine mesenchymal stem cells, and were able to transfect human breast cancer cells. Overall, this set of data pre-validates the use of degradable DHBC as non-viral vectors for the encapsulation and the controlled release of siRNA, which may therefore constitute a sound alternative to non-degradable and/or cytotoxic polycationic vectors.
Direct synthesis of peptide-containing silicone. A new way for bioactive materials
This is custom heading element
Chem. Eur. J. 10.1002/chem.202001571
Ahmad Mehdi, Martin Julie, Mohammad Wehbi, Cecile Echalier, Sylvie hunger, Audrey Bethry, Xavier garric, coline Pinese, jean Martinez, Lubomir vezenkov, and gilles subra
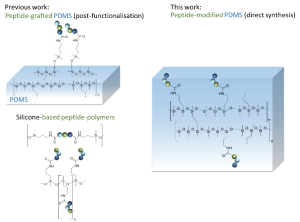
ABSTRACT
A simple and efficient way to synthesize peptide-containing silicone materials is described. Silicone oils containing a chosen ratio of bioactive peptide sequences were prepared by acid-catalyzed copolymerization of dichlorodimethylsilane, hybrid dichloromethyl peptidosilane and either Si-vinyl or Si-H functionalized monomers. Functionalized silicone oils were first obtained and then after hydrosilylation cross-linking, bioactive PDMS based materials were straightforward obtained. The introduction of an antibacterial peptide yields PDMS materials showing an interesting activity against Staphylococcus Aureus. In the same way, RGD ligands-containing PDMS demonstrated improved cell adhesion properties. This generic method was fully compatible with the stability of peptides and thus opened the way to the synthesis of a wide range of biologically active silicones.
Biomimicking Fiber Platform with Tunable Stiffness to Study Mechanotransduction Reveals Stiffness Enhances Oligodendrocyte Differentiation but Impedes Myelination through YAP‐Dependent Regulation
This is custom heading element
William Ong, Nicolas Marinval, Junquan Lin, Mui Hoon Nai, Yee-Song Chong, Coline Pinese, Sreedharan Sajikumar, Chwee Teck Lim, Charles Ffrench-Constant, Marie E. Bechler, and Sing Yian Chew
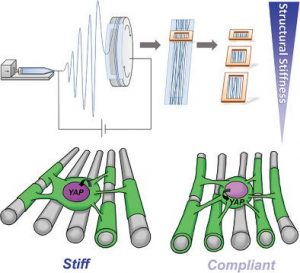
ABSTRACT
A key hallmark of many diseases, especially those in the central nervous system (CNS), is the change in tissue stiffness due to inflammation and scarring. However, how such changes in microenvironment affect the regenerative process remains poorly understood. Here, a biomimicking fiber platform that provides independent variation of fiber structural and intrinsic stiffness is reported. To demonstrate the functionality of these constructs as a mechanotransduction study platform, these substrates are utilized as artificial axons and the effects of axon structural versus intrinsic stiffness on CNS myelination are independently analyzed. While studies have shown that substrate stiffness affects oligodendrocyte differentiation, the effects of mechanical stiffness on the final functional state of oligodendrocyte (i.e., myelination) has not been shown prior to this. Here, it is demonstrated that a stiff mechanical microenvironment impedes oligodendrocyte myelination, independently and distinctively from oligodendrocyte differentiation. Yes-associated protein is identified to be involved in influencing oligodendrocyte myelination through mechanotransduction. The opposing effects on oligodendrocyte differentiation and myelination provide important implications for current work screening for promyelinating drugs, since these efforts have focused mainly on promoting oligodendrocyte differentiation. Thus, the platform may have considerable utility as part of a drug discovery program in identifying molecules that promote both differentiation and myelination.
Turning peptides into bioactive nylons
This is custom heading element
Eur. Polym. J. 2020, 135, 109886.
Said Jebors*, Coline Pinese*, Titouan Montheil*, Audrey Bethry, Simon Verquin, Louise Plais, Marie Moulin, Chloé Dupont, Xavier Garric, Ahmad Mehdi, Jean Martinez, Gilles Subra
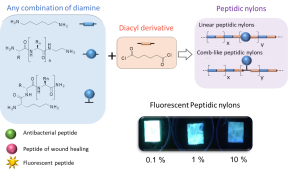
ABSTRACT
New synthetic textiles with physical and/or biological properties are increasingly used in medical applications. While a simple textile coating is usually carried out to obtain biological properties, covalent grafting should be considered for long-term applications. Herein, we have developed a new hybrid bioactive nylon whose synthesis involves a peptide sequence with a diacyl derivative. Numerous types of peptide-nylons were prepared by varying the molar percentage (0.1 %, 1 % and 10%) and orientation of the peptide in the polymer backbone. Nylons incorporating antibacterial peptides significantly inhibited S. aureus proliferation whereas nylons functionalized with cell-adhesive peptide enhanced the proliferation of L929 fibroblast. These results show that the incorporation of the peptides directly into the nylon skeleton is efficient and provides biological properties that suggest new ways of functionalizing biomedical textiles.
Graft Copolymers with Tunable Amphiphilicity tailored for Efficient Dual Drug Delivery via Encapsulation and pH-sensitive Drug Conjugation
This is custom heading element
Polymer Chemistry 11, 4438–4453 (2020)
Bláhová M., Randárová E., Konefał R., Nottelet B., Etrych T.
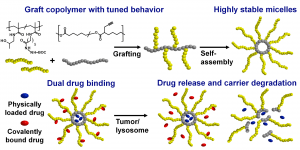
ABSTRACT
Polymer-based drug delivery systems may significantly improve cancer therapy. We developed amphiphilic poly(e-caprolactone)-graft-(poly-N-(2-hydroxypropyl) methacrylamide) copolymers (PCL-graft-pHPMA) with tunable amphiphilicity intended for efficient dual delivery via simultaneous encapsulation of hydrophobic drug, Bcl-2 inhibitor ABT-199, and pH-sensitive conjugation of other chemotherapeutics, doxorubicin, to desired sites, e.g. tumors. Using controlled RAFT polymerization and click chemistry well-defined PCL-graft-pHPMA of diverse Mw and physical properties were prepared. By simple dissolution they self-assembled into highly stable micelles with Dh ≈ 25 nm and low critical micelle concentration (around 5 μg mL-1). The total drug payload reached 17 wt % while maintaining system solubility. The micelles exhibited long-term stability in buffers, while they were cleaved in the presence of lipase, thus proving degradation and drug release after uptake to lysosomes of cancer cells with minimal drug leakage during blood circulation. PCL-graft-pHPMA micelles may serve as a long-circulating drug depo for effective dual therapy of diverse malignancies.
In Vivo Tissue-Engineered Vascular Grafts
This is custom heading element
Tissue-Engineered Vascular Grafts, Reference Series in
Biomedical Engineering
Walpoth B.H., de Valence S., Tille J-C., Mugnai D., Sologashvili T., Mrówczyński W.,
Cikirikcioglu M., Pektok E., Osorio S., Innocente F., Bochaton-Piallat M-L., Nottelet B., Kalangos A., Gurny R.
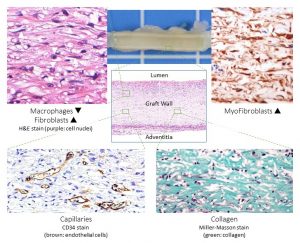
ABSTRACT
Vascular grafts are needed for coronary and peripheral vascular bypass surgeries as well as for access surgeries for hemodialysis and reconstruction of congenital heart defects. Despite good results in the large caliber, small caliber (<6 mm) show unsatisfactory clinical results. Tissue-engineered vascular grafts (TEVG) have been made using several approaches ranging from acellular synthetic or biologic polymer scaffolds to decellularized natural matrices, self-assembled cell-based bioreactor matured, or 3D cell-printed constructs. This chapter will focus mainly on in vivo tissue engineering which was used as first-in-man. This is based on an acellular, synthetic, degradable, polymer scaffold which is repopulated by the host cells after implantation to create a “neo-artery.” Advantages are shelf-readiness; simple, costeffective manufacturing; and avoidance of bioreactor cell maturation. Short-, mid-, and long-term experimental and clinical results show good cellular remodeling with extracellular matrix formation and endothelialization as well as patency and function. Thus, the approach of using an acellular, synthetic, biodegradable scaffold is an optimal clinical option for TEVG.
Synergistic Anti-fouling and Bactericidal Poly(ether ether ketone) Surfaces via a One-step Photomodification
This is custom heading element
Mater Sci Eng C. 111,110811 (2020)
Buwalda S., Rotman S., Eglin D., Moriarty F., Bethry A., Garric X., Guillaume O., Nottelet B.
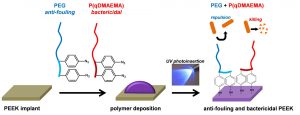
ABSTRACT
Implants of poly(ether ether ketone) (PEEK) are gaining importance in surgical bone reconstruction of the skull. As with any implant material, PEEK is susceptible to bacterial contamination and occasionally PEEK implants were removed from patients because of infection. To address this problem, a combination of anti-fouling and bactericidal polymers are grafted onto PEEK. The originality is that anti-fouling (modified poly(ethylene glycol)) and bactericidal (quaternized poly(dimethylaminoethyl acrylate)) moieties are simultaneously and covalently grafted onto PEEK via UV photoinsertion. The functionalized PEEK surfaces are evaluated by water contact angle measurements, FTIR, XPS and AFM. Grafting of anti-fouling and bactericidal polymers significantly reduces Staphylococcus aureus adhesion on PEEK surfaces without exhibiting cytotoxicity in vitro. This study demonstrates that grafting combinations of anti-fouling and bactericidal polymers synergistically prevents bacterial adhesion on PEEK implants. This approach shows clinical relevance as grafting is rapid, does not modify PEEK properties and can be conducted on pre-formed implants.
Role of Polymer Micelles in the Delivery of Photodynamic Therapy Agent to Liposomes and Cells
This is custom heading element
Gibot L., Demazeau M., Pimienta V., Mingotaud A-F., Vicendo P., Collin F., Martins-Froment N., Dejean S., Nottelet B., Roux C.,Lonetti B.
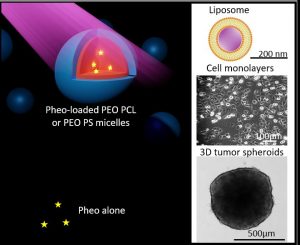
ABSTRACT
The use of nanocarriers for hydrophobic photosensitizers in the context of photodynamic therapy (PDT) to improve pharmacokinetics and biodistribution is well established. However, the mechanisms at play in the internalization of nanocarriers are not well elucidated despite being crucial to inspiring nanocarrier design. Here we focus on the mechanisms involved in copolymer PEO-PCL and PEO-PS micelles – membrane interactions through complementary physico-chemical studies on biomimetic membranes and biological experiments on 2D and 3D cell cultures. Förster Resonance Energy Transfer measurements on fluorescently labelled lipid vesicles and flow cytometry on two cancerous cell lines allowed evaluation of the uptake of a photosensitizer, Pheophorbide a (Pheo), and copolymer chains towards model membranes and cells respectively. The effects of calibrated light illumination for PDT treatment on lipid vesicle membranes, i.e. leakage and formation of oxidized lipids, and cell viability, were assessed. No significant differences were observed between the ability of PEO-PCL and PEO-PS micelles to deliver Pheo to model membranes, but Pheo was found in higher concentrations in cells in the case of PEO-PCL. These higher Pheo concentrations did not correspond to better performances in PDT treatment. We thus highlighted subtle differences in PEO-PCL and PEO-PS micelles for the delivery of Pheo.
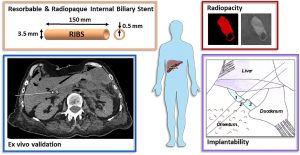
From in vitro evaluation to human post-mortem pre-validation of a radiopaque and resorbable internal biliary stent for liver transplantation applications
This is custom heading element
Acta Biomaterialia 106, 66-81, (2020)
Girard E., Chagnon G., Broisat A., Dejean S., Soubies A., Gil H., Sharkawi T., Boucher F. Roth G.S., Trilling B., Nottelet B.
ABSTRACT
The implantation of an internal biliary stent (IBS) during liver transplantation has recently been shown to reduce biliary complications. To avoid a potentially morbid ablation procedure, we developed a resorbable and radiopaque internal biliary stent (RIBS). We studied the mechanical and radiological properties of RIBS upon in vivo implantation in rats and we evaluated RIBS implantability in human anatomical specimens.
For this purpose, a blend of PLA50-PEG-PLA50 triblock copolymer, used as a polymer matrix, and of X-ray-visible triiodobenzoate-poly(e-caprolactone) copolymer (PCL-TIB), as a radiopaque additive, was used to design X-ray-visible RIBS. Samples were implanted in the peritoneal cavity of rats. The radiological, chemical, and biomechanical properties were evaluated during degradation. Further histological studies were carried out to evaluate the degradation and compatibility of the RIBS. A human cadaver implantability study was also performed.
The in vivo results revealed a decline in the RIBS mechanical properties within 3 months, whereas clear and stable X-ray visualization of the RIBS was possible for up to 6 months. Histological analyses confirmed compatibility and resorption of the RIBS, with a limited inflammatory response. The RIBS could be successfully implanted in human anatomic specimens. The results reported in this study will allow the development of trackable and degradable IBS to reduce biliary complications after liver transplantation.
Performances and behavior of a water-soluble and pH-sensitive polycarboxybetaine used for metal ion recovery
This is custom heading element
Materials Today Communications 20, 100575, 2019
Mouton, J., Kirkelund, G.M., Hassen, Y., Chastagnol, S., Van den Berghe, H., Coudane, J., Turmine, M
ABSTRACT
Zwiterionic functional groups give polycarboxybetaines a great ability to chelate copper and their use for metal ions recovery was suggested in previous studies. The use of a water-soluble and pH-sensitive polymer (polycarboxy-ethyl-3-aminocrotonate – PCEAC) was investigated for the removal of copper from aqueous solution. The equilibrium adsorption level was determined as a function of temperature, pH and initial adsorbate concentration. The good fit to the Langmuir model offered various information about copper uptake mechanisms. The maximal adsorption capacities were found to reach 253 ± 11 mg of copper per g of PCEAC at pH=6. The thermodynamic parameters such as free energy, enthalpy and entropy changes for the adsorption of copper were computed to predict the nature of adsorption process. The removal of copper was varying from 2% to 97% depending on pH and initial copper concentration. A particular behavior of PCEAC at pH=4 is discussed and explained by viscosity measurements as a particular conformation of the polymer. The effect of cadmium on copper adsorption was also tested. Some co-adsorption phenomena were observed at low cadmium concentrations ([Cd]/[Cu]≤1), while copper desorption in favor of cadmium adsorption were quantified at higher cadmium concentrations ([Cd]/[Cu]>1).
Double-hydrophilic block copolymers based on functional poly(ε-caprolactone)s for pH-dependent controlled drug delivery
This is custom heading element
Biomacromolecules 21, 397, (2020)
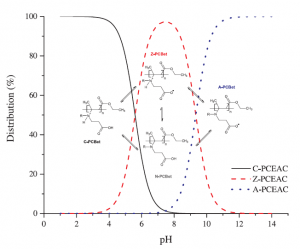
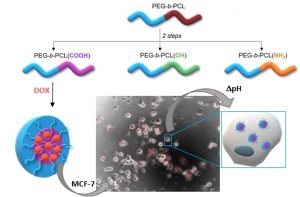
Ayman El Jundi, Sytze Buwalda, Audrey Bethry, Sylvie Hunger, Jean Coudane, Youssef Bakkour, Benjamin Nottelet
ABSTRACT
The use of double-hydrophilic block copolymers (DHBCs) in biomedical applications is limited by their lack of degradability. This additional functionality has been obtained in the past through multistep chemical strategies associated with low yields. In this work, a series of DHBCs composed of a bioeliminable poly(ethylene glycol) (PEG) block and hydrolysable functional poly(e-caprolactone) (PCL) blocks bearing carboxylic (PEG-b-PCL(COOH)), amino (PEG-b-PCL(NH2)) or hydroxyl side groups (PEG-b-PCL(OH)) is synthesized in only 3 steps. DHBCs with 50% substitution degree with respect to the CL units are obtained for all functional groups. The pH-dependent self-assembly behavior of the DHBCs is studied showing critical micelle concentration (CMC) variations by a factor 2 upon pH changes and micellar mean diameter variations of 20-30%. The potential of these partly degradable DHBCs as drug-loaded polyion complex micelles is further exemplified with the PEG-b-PCL(COOH) series that is associated with the positively charged anticancer drug doxorubicin (DOX). Encapsulation efficiencies, drug loadings, pH-controlled release and cytotoxicity of the DOX-loaded micelles towards cancer cells are demonstrated. This set of data confirms the interest of the proposed straightforward chemical strategy to generate fully bioeliminable and partly degradable DHBCs with potential as pH-responsive drug delivery systems.
Preliminary design of a new degradable medical device to prevent the formation and recurrence of intrauterine adhesions
This is custom heading element
Communications Biology 2, 196, (2019)
Leprince, S., Huberlant, S., Allegre, L., Warembourg, S., Leteuff, I., Bethry, A., Paniagua, C., Taillades, H., Tayrac, R. D., Coudane, J., Letouzey, V. & Garric, X.
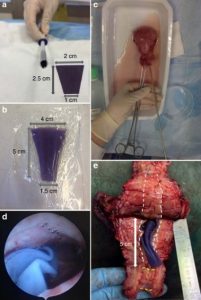
ABSTRACT
Intrauterine adhesions lead to partial or complete obliteration of the uterine cavity and have life-changing consequences for women. The leading cause of adhesions is believed to be loss of stroma resulting from trauma to the endometrium after surgery. Adhesions are formed when lost stroma is replaced by fibrous tissue that join the uterine walls. Few effective intrauterine anti-adhesion barriers for gynecological surgery exist. We designed a degradable anti-adhesion medical device prototype to prevent adhesion formation and recurrence and restore uterine morphology. We focused on ideal degradation time for complete uterine re-epithelialization for optimal anti-adhesion effect and clinical usability. We developed a triblock copolymer prototype [poly(lactide) combined with high molecular mass poly(ethylene oxide)]. Comparative pre-clinical studies demonstrated in vivo anti-adhesion efficacy. Ease of introduction and optimal deployment in a human uterus confirmed clinical usability. This article provides preliminary data to develop an intrauterine medical device and conduct a clinical trial.
Biomechanical behaviour of human bile duct wall and impact of cadaveric preservation processes.
This is custom heading element
J. Mech. Behav. Biomed. 98, 291–300 (2019)
Girard E., Chagnon G., Gremen E., Calvez M., Boutonnat J., Trilling B., Nottelet B.
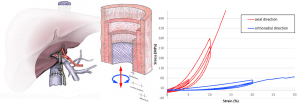
ABSTRACT
Biliary diseases are the third most common cause of surgical digestive disease. There is a close relationship between the mechanical performance of the bile duct and its physiological function. Data of biomechanical properties of human main bile duct are scarce in literature. Furthermore, mechanical properties of soft tissues are affected by these preservation procedures. The aim of the present work was, on the one hand, to observe the microstructure of the human bile duct by means of histological analysis, on the other hand, to characterize the mechanical behavior and describe the impact of different preservation processes. A mechanical study in a controlled environment consisting of cyclic tests was made. The results of the mechanical tests are discussed and explained using the micro-structural observations. The results show an influence of the loading direction, which is representative of an anisotropic behavior. A strong hysteresis due to the viscoelastic properties of soft tissues was also observed. Embalming and freezing preservation methods had an impact on the biomechanical properties of human main bile duct, with fiber network deterioration. That may further provide a useful quantitative baseline for anatomical and surgical training using embalming and freezing.
Biomimicking Fiber Scaffold as an Effective In Vitro and In Vivo MicroRNA Screening Platform for Directing Tissue Regeneration
This is custom heading element
Na Zhang, Ulla Milbreta, Jiah Shin Chin, Coline Pinese, Junquan Lin, Hitomi Shirahama, Wei Jiang, Hang Liu, Ruifa Mi, Ahmet Hoke, Wutian Wu and Sing Yian Chew

ABSTRACT
MicroRNAs effectively modulate protein expression and cellular response. Unfortunately, the lack of robust nonviral delivery platforms has limited the therapeutic application of microRNAs. Additionally, there is a shortage of drug‐screening platforms that are directly translatable from in vitro to in vivo. Here, a fiber substrate that provides nonviral delivery of microRNAs for in vitro and in vivo microRNA screening is introduced. As a proof of concept, difficult‐to‐transfect primary neurons are targeted and the efficacy of this system is evaluated in a rat spinal cord injury model. With this platform, enhanced gene‐silencing is achieved in neurons as compared to conventional bolus delivery (p < 0.05). Thereafter, four well‐recognized microRNAs (miR‐21, miR‐222, miR‐132, and miR‐431) and their cocktails are screened systematically. Regardless of age and origin of the neurons, similar trends are observed. Next, this fiber substrate is translated into a 3D system for direct in vivo microRNA screening. Robust nerve ingrowth is observed as early as two weeks after scaffold implantation. Nerve regeneration in response to the microRNA cocktails is similar to in vitro experiments. Altogether, the potential of the fiber platform is demonstrated in providing effective microRNA screening and direct translation into in vivo applications.
Scaffold-Mediated Sustained, Non-viral Delivery of miR-219/miR-338 Promotes CNS Remyelination
This is custom heading element
Ulla Milbreta, Junquan Lin, Coline Pinese, William Ong, Jiah Shin Chin, Hitomi Shirahama, Ruifa Mi, Anna Williams, Marie E. Bechler, Jun Wang, Charles french-Constant, Ahmet Hoke and Sing Yian Chew
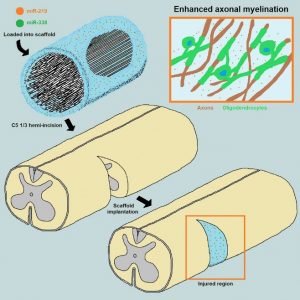
ABSTRACT
The loss of oligodendrocytes (OLs) and subsequently myelin sheaths following injuries or pathologies in the CNS leads to debilitating functional deficits. Unfortunately, effective methods of remyelination remain limited. Here, we present a scaffolding system that enables sustained non-viral delivery of microRNAs (miRs) to direct OL differentiation, maturation, and myelination. We show that miR-219/miR-338 promoted primary rat OL differentiation and myelination in vitro. Using spinal cord injury as a proof-of-concept, we further demonstrate that miR-219/miR-338 could also be delivered non-virally in vivo using an aligned fiber-hydrogel scaffold to enhance remyelination after a hemi-incision injury at C5 level of Sprague-Dawley rats. Specifically, miR-219/miR-338 mimics were incorporated as complexes with the carrier, TransIT-TKO (TKO), together with neurotrophin-3 (NT-3) within hybrid scaffolds that comprised poly(caprolactone-co-ethyl ethylene phosphate) (PCLEEP)-aligned fibers and collagen hydrogel. After 1, 2, and 4 weeks post-treatment, animals that received NT-3 and miR-219/miR-338 treatment preserved a higher number of Olig2+ oligodendroglial lineage cells as compared with those treated with NT-3 and negative scrambled miRs (Neg miRs; p < 0.001). Additionally, miR-219/miR-338 increased the rate and extent of differentiation of OLs. At the host-implant interface, more compact myelin sheaths were observed when animals received miR-219/miR-338. Similarly within the scaffolds, miR-219/miR-338 samples contained significantly more myelin basic protein (MBP) signals (p < 0.01) and higher myelination index (p < 0.05) than Neg miR samples. These findings highlight the potential of this platform to promote remyelination within the CNS.
Scaffold-mediated sequential drug/gene delivery to promote nerve regeneration and remyelination following traumatic nerve injuries
This is custom heading element
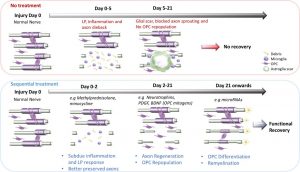
Ong, W., Pinese, C. & Chew, S. Y.
ABSTRACT
Stem cells combined with biodegradable injectable scaffolds releasing growth factors hold great promises in regenerative medicine, particularly in the treatment of neurological disorders. We here integrated human marrow-isolated adult multilineage-inducible (MIAMI) stem cells and pharmacologically active microcarriers (PAMs) into an injectable non-toxic silanized-hydroxypropyl methylcellulose (Si-HPMC) hydrogel. The goal is to obtain an injectable non-toxic cell and growth factor delivery device. It should direct the survival and/or neuronal differentiation of the grafted cells, to safely transplant them in the central nervous system, and enhance their tissue repair properties. A model protein was used to optimize the nanoprecipitation conditions of the neuroprotective brain-derived neurotrophic factor (BDNF). BDNF nanoprecipitate was encapsulated in fibronectin-coated (FN) PAMs and the in vitro release profile evaluated. It showed a prolonged, bi-phasic, release of bioactive BDNF, without burst effect. We demonstrated that PAMs and the Si-HPMC hydrogel increased the expression of neural/neuronal differentiation markers of MIAMI cells after 1 week. Moreover, the 3D environment (PAMs or hydrogel) increased MIAMI cells secretion of growth factors (b-NGF, SCF, HGF, LIF, P1GF-1, SDF-1 alpha, VEGF-A & D) and chemokines (MIP-la & RANTES, IL-8). These results show that PAMs delivering BDNF combined with Si-HPMC hydrogel represent a useful novel local delivery tool in the context of neurological disorders. It not only provides neuroprotective BDNF but also bone marrow-derived stem cells that benefit from that environment by displaying neural commitment and an improved neuroprotective/reparative secretome. It provides preliminary evidence of a promising pro-angiogenic, neuroprotective and axonal growth-promoting device for the nervous system. Statement of Significance Combinatorial tissue engineering strategies for the central nervous system are scarce. We developed and characterized a novel injectable non-toxic stem cell and protein delivery system providing regenerative cues for central nervous system disorders. BDNF, a neurotrophic factor with a wide-range effect, was nanoprecipitated to maintain its structure and released in a sustained manner from novel polymeric microcarriers. The combinatorial 3D support, provided by fibronectin-microcarriers and the hydrogel, to the mesenchymal stem cells guided the cells towards a neuronal differentiation and enhanced their tissue repair properties by promoting growth factors and cytokine secretion. The long-term release of physiological doses of bioactive BDNF, combined to the enhanced secretion of tissue repair factors from the stem cells, constitute a promising therapeutic approach.
Degradable multi(aryl-azide) star copolymer as universal photo-crosslinker for elastomeric scaffolds
This is custom heading element
Mater. Today Chem. 12, 209-221, (2019)
Gangolphe L., Déjean S., Bethry A., Hunger S., Pinese C., Garric X., Bossard F., Nottelet B.
ABSTRACT
Degradable elastomers with elastic properties close to those of soft-tissues are necessary for tissue-engineering. Most degradable elastomers developed so far are based on functional low molecular weight pre-polymers that are combined with molecular crosslinkers to yield the elastomeric 3D networks. To overcome this limitation, we present in this work the concept of star-shaped macromolecular multi(aryl-azide) photo-crosslinker that has the ability to efficiently crosslink any polymer containing C-H bonds independently of its molecular weight and without the need for pre-functionalization. This concept of universal crosslinking agent is illustrated with a star-shaped block copolymer composed of an 8-arm poly(ethylene glycol) core and poly(lactide) side arms functionalized with aryl-azide moieties (PEG8arm-PLA-fN3). It was selected due to its macromolecular nature that allows for an easy processing of electrospun photo-crosslinked scaffolds while making it possible to adapt its chemical nature with the one of the polymer matrix. A parameter study is first carried out on PEG8arm-PLA-fN3 / PLA-Pluronic®-PLA films to evaluate the impact of the polymers molecular weight, PEG/PLA ratios, and UV irradiation conditions on the crosslinking efficiency. This study confirms that high crosslinking efficiencies can be obtained with PEG8arm-PLA-fN3 (60%) compared to commercially availabe bis(aryl-azide) photo-crosslinker (below 15%). Optimal conditions are then used to yield electrospun microfibers (1-2 µm) crosslinked with PEG8arm-PLA-fN3 resulting in biocompatible and highly elastomeric scaffolds (ε_y>100%) compared to uncrosslinked scaffolds(ε_y<10%). In addition, we show that the degradation rate can be controlled over time depending on the blend content of PEG8arm-PLA-fN3. Taken together, these results demonstrate the potential of macromolecular multi(aryl-azide) photo-crosslinkers to develop original degradable elastomeric scaffolds for soft-tissue reconstruction.
Controlled Anchoring of Iron Oxide Nanoparticles on Polymeric Nanofibers: Easy Access to Core@Shell Organic−Inorganic Nanocomposites for Magneto-Scaffolds
This is custom heading element
ACS Appl. Mater. Interfaces 11, 9519–9529 (2019)
Awada H., Al Samad A., Laurencin D., Gilbert R., Dumail X., El Jundi A., Bethry A., Pomrenke R., Johnson C., Lemaire L., Franconi F., Félix G., Larionova J., Guari Y., Nottelet B.

ABSTRACT
Composites combining superparamagnetic iron oxide nanoparticles (SPIONs) and polymers are largely present in modern (bio)materials. However, while SPIONs embedded in polymer matrices are classically reported, the mechanical and degradation properties of the polymer scaffold are impacted by the SPIONs. Therefore, the controlled anchoring of SPIONs onto polymer surfaces is still a major challenge. Herein, we propose an efficient strategy for the direct and uniform anchoring of SPIONs on the surface of functionalized-polylactide (PLA) nanofibers via a simple free ligand exchange procedure to design PLA@SPIONs core@shell nanocomposites. The resulting PLA@SPIONs hybrid biomaterials are characterized by electron microscopy (SEM and TEM) and EDXS analysis, to probe the morphology and detect elements present at the organic/inorganic interface, respectively. A monolayer of SPIONs with a complete and homogeneous coverage is observed on the surface of PLA nanofibers. Magnetization experiments show that magnetic properties of the nanoparticles are well-preserved after their grafting on the PLA fibers and that the size of the nanoparticles does not change. The absence of cytotoxicity, combined with a high sensitivity of detection in MRI both in vitro and in vivo make these hybrid nanocomposites attractive for the development of magnetic biomaterials for biomedical applications.
UV-triggered photoinsertion of contrast agent onto polymer surfaces for in vivo MRI-visible medical devices
This is custom heading element
Multifunct. Mater. 2 0240012019 (2019)
Schulz A., Lemaire L., Bethry A.; Allegre L., Cardoso M., Bernex F., Franconi F., Goze-Bac C., Taillades H., Garric X., Nottelet B.
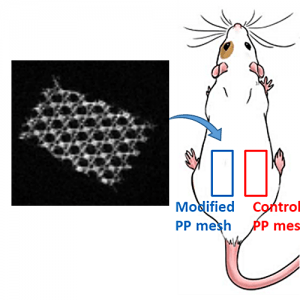
ABSTRACT
Polymeric materials are largely employed for the manufacturing of implants for various reasons, but they are typically invisible by conventional imaging methods. To improve surgical procedure and postoperative implant follow-up though, biomaterials are needed which allow an accurate and efficient imaging. Here, we present a direct and versatile strategy that allows to covalently immobilize T1 magnetic resonance imaging (MRI) contrast agents at the surface of various clinically relevant polymeric biomaterials. An aryl-azide bearing complex of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and gadolinium (Gd) has been synthesized for easy photografting onto polymer surfaces. Polycaprolactone (PCL), polylactide (PLA), polyurethane (PU), polyetheretherketone (PEEK) and polypropylene (PP) have been selected as clinically relevant substrates and successfully functionalized with the photosensitive MRI probe DOTA/Gd. Following in vitro assessment of their biocompatibility and MRI visibility, commercial MRI-visible PP hernia repair meshes (MRI-meshes) have been prepared. MRI-meshes have been implanted in rats for in vivo evaluation of their imaging capacities over 1 month. Histological evaluation and Gd biodistribution studies have been carried out confirming the potential of this straightforward approach to simply yield imageable medical devices.
Ultrafast in situ forming poly(ethylene glycol)-poly(amido amine) hydrogels with tunable drug release properties via controllable degradation rates
This is custom heading element
Eur. J. Pharm. Biopharm. 139, 232-239 (2019)
Buwalda S., Bethry A., Hunger S., Kandoussi S., Coudane J., Nottelet B.
ABSTRACT
Fast in situ forming, chemically crosslinked hydrogels were prepared by the amidation reaction between N-succinimidyl ester end groups of multi-armed poly(ethylene glycol) (PEG) and amino surface groups of poly(amido amine) (PAMAM) dendrimer generation 2.0. To control the properties of the PEG/PAMAM hydrogels, PEGs were used with different arm numbers (4 or 8) as well as different linkers (amide or ester) between the PEG arms and their terminal N-succinimidyl ester groups. Oscillatory rheology measurements showed that the hydrogels form within seconds after mixing the PEG and PAMAM precursor solutions. The storage moduli increased with crosslink density and reached values up to 2.3 kPa for hydrogels based on 4-armed PEG. Gravimetrical degradation experiments demonstrated that hydrogels with ester linkages between PEG and PAMAM degrade within 2 days, whereas amide-linked hydrogels were stable for several months. The release of two different model drugs (fluorescein isothiocyanate-dextran with molecular weights of 4·103 and 2·106 g/mol, FITC-DEX4K and FITC-DEX2000K, respectively) from amide-linked hydrogels was characterized by an initial burst followed by diffusion-controlled release, of which the rate depended on the size of the drug. In contrast, the release of FITC-DEX2000K from ester-containing hydrogels was governed mainly by degradation of the hydrogels and could be modulated via the ratio between ester and amide linkages. In vitro cytotoxicity experiments indicated that the PEG/PAMAM hydrogels are non-toxic to mouse fibroblasts. These in situ forming PEG/PAMAM hydrogels can be tuned with a broad range of mechanical, degradation and release properties and therefore hold promise as a platform for the delivery of therapeutic agents.
Interaction of gentamicin sulfate with alginate and consequences on the physico-chemical properties of alginate-containing biofilms
This is custom heading element
Int. J. Biol. Macromol. 121, 390–397 (2019)
Heriot, M., Nottelet, B., Garric, X., D’Este, M., Richards, G. R., Moriarty, F. T., Eglin, D. & Guillaume, O.
ABSTRACT
Background: Alginate is one of the main extracellular polymeric substances (EPS) in biofilms of Cystic Fibrosis (CF) patients suffering frompulmonary infections. Gentamicin sulfate (GS) can strongly bind to alginate resulting in loss of pharmacological activity; however neither the mechanism nor its repercussion is fully understood. In this study, we investigated how GS modifies the alginate macromolecular network and its microenvironment. Material and methods: Alginate gels of two different compositions (either enriched in guluronate units (G) or enriched inmannuronate units(M))were crosslinkedwith Ca2+ and exposed to GS at varying times and concentrations.
The complexes formed were characterized via turbidimetry, mechanical tests, swelling assay, calorimetry techniques, nuclear magnetic resonance, Ca2+ displacement, macromolecular probe diffusion and pH alteration.
Results: In presence of GS, the alginate network and its environment undergo a tremendous reorganization in terms of gel density, stiffness, diffusion property, presence and state of the water molecules. We noted that the intensity of those alterations is directly dependent on the polysaccharide motif composition (ratio M/G).
Conclusion: Our results underline the importance of alginate as biofilm component, its pernicious role during antibiotherapy and could represent a potential macromolecular target to improve anti-infectious
therapies.
Phthalocyanine photosensitizer in polyethylene glycol-block-poly(lactide-co-benzyl glycidyl ether) nanocarriers: Probing the contribution of aromatic donor-acceptor interactions in polymeric nanospheres
This is custom heading element
Mater. Sci. Eng. C-Mater. Biol. Appl. 94, 220–233 (2019)
Pound-Lana, G. E. N., Garcia, G. M., Trindade, I. C., Capelari-Oliveira, P., Pontifice, T. G., Vilela, J. M. C., Andrade, M. S., Nottelet, B., Postacchini, B. B. & Mosqueira, V. C. F.
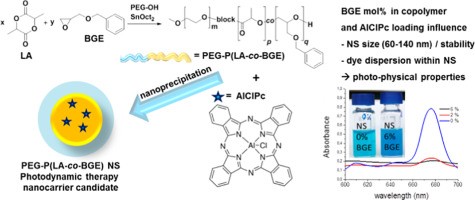
ABSTRACT
For best photosensitizer activity phthalocyanine dyes used in photodynamic therapy should be molecularly dispersed. Polyethylene glycol-block-polylactide derivatives presenting benzyl side-groups were synthesized to encapsulate a highly lipophilic phthalocyanine dye (AlClPc) and evaluate the effect of π-π interactions on the nanocarrier colloidal stability and dye dispersion. Copolymers with 0, 1, 2 and 6mol% of benzyl glycidyl ether (BGE) were obtained via polyethylene glycol initiated ring-opening copolymerization of D,l-lactide with BGE. The block copolymers formed stable, monodisperse nanospheres with low in vitro cytotoxicity. AlClPc loading increased the nanosphere size and affected their colloidal stability. The photo-physical properties of the encapsulated dye, studied in batch and after separation by field flow fractionation, demonstrated the superiority of plain PEG-PLA over BGE-containing copolymers in maintaining the dye in its monomeric (non-aggregated) form in aqueous suspension. High dye encapsulation and sustained dye release suggest that these nanocarriers are good candidates for photodynamic therapy.
Reversibly core-crosslinked PEG-P(HPMA) micelles: Platinum coordination chemistry for competitive-ligand-regulated drug delivery
This is custom heading element
J. Colloid Interface Sci. 535, 505–515 (2019)
Buwalda, S., Nottelet, B., Bethry, A., Kok, R. J., Sijbrandi, N. & Coudane, J.
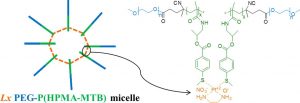
ABSTRACT
Hypothesis. The presence of pendant thioether groups on poly(ethylene glycol)-poly(N(2-hydroxypropyl) methacrylamide) (PEG-P(HPMA)) block copolymers allows for platinum-mediated coordinative micellar core-crosslinking, resulting in enhanced micellar stability and stimulus-responsive drug delivery.
Experiments. A new PEG-P(HPMA) based block copolymer with pendant 4-(methylthio)benzoyl (MTB) groups along the P(HPMA) block was synthesized by free radical polymerization of a novel HPMA-MTB monomer using a PEG based macro-initiator. As crosslinker the metal-organic linker [ethylenediamineplatinum(II)]2+ was used, herein called Lx, which is a coordinative linker molecule that has been used for the conjugation of drug molecules to a number of synthetic or natural carrier systems such as hyperbranched polymers and antibodies.
Findings. The introduction of Lx in the micellar core results in a smaller size, a lower critical micelle concentration and a better retention of the hydrophobic drug curcumin thanks to coordination bonds between the central platinum atom of Lx and thioether groups on different polymer chains. The drug release from Lx crosslinked micelles is significantly accelerated under conditions mimicking the intracellular environment due to competitive coordination and subsequent micellar de-crosslinking. Because of their straightforward preparation and favorable drug release characteristics, core-crosslinked Lx PEG-P(HPMA) micelles hold promise as a versatile nanomedicine platform.
Iterative Photoinduced Chain Functionalization as a Generic Platform for Advanced Polymeric Drug Delivery Systems
This is custom heading element
Macromol. Rapid Commun. 39, 1700502 (2018)
Al Samad, A., Bethry, A., Janouskova, O., Ciccione, J., Wenk, C., Coll, J.-L., Subra, G., Etrych, T., El Omar, F., Bakkour, Y., Coudane, J. & Nottelet, B.
ABSTRACT
Advanced drug delivery systems (DDS) are easily designed following a photoiterative strategy. Multifunctional polymers are obtained by coupling building blocks of interest to an alkynated poly(ε‐caprolactone) (PCL) platform via an efficient thiol–yne photoaddition. Fine‐tuning over the design is achieved, as illustrated with targeting and enzyme‐responsive DDS.
Stabilization of poly(ethylene glycol)-poly(epsilon-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties
This is custom heading element
Colloid Interface Sci. 514, 468–478 (2018)
Buwalda, S., Al Samad, A., El Jundi, A., Bethry, A., Bakkour, Y., Coudane, J. & Nottelet, B
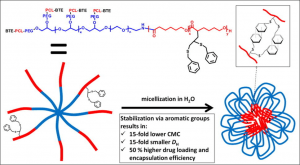
ABSTRACT
Hypothesis
The functionalization of poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) block copolymers with moieties allowing for core-crosslinking is expected to result in improved micellar stability and drug delivery properties.
Experiments
PEG-(PCL)8 star block copolymers were functionalized with pendant benzylthioether (BTE) groups by applying an anionic post-polymerization modification technique followed by photoradical thiol-yne addition of benzyl mercaptan. The micellar properties of PEG-(PCL)8 and PEG-(PCL-BTE)8 were studied and compared in terms of critical micelle concentration (CMC), size, morphology, drug loading and release and in vitro cytotoxicity.
Findings
In comparison with unmodified PEG-(PCL)8 micelles, PEG-(PCL-BTE)8 micelles exhibited a 15-fold lower CMC, a 15-fold smaller size and a 50% higher drug loading and encapsulation efficiency thanks to the presence of pendant benzyl groups which provide the possibility for micellar core-crosslinking via supramolecular π-π stacking and additional hydrophobic interactions. Whereas the PEG-(PCL)8 micelles showed significant aggregation during in vitro cytotoxicity experiments, the PEG-(PCL-BTE)8 micelles showed no signs of aggregation and were capable of solubilizing high concentrations of curcumin, resulting in a significant decrease in MCF-7 cell viability after 48 h. Their ease of synthesis combined with promising results regarding drug delivery make the PEG-(PCL-BTE)8 micelles appealing for application in the field of encapsulation.
Direct Photomodification of Polymer Surfaces: Unleashing the Potential of Aryl-Azide Copolymers
This is custom heading element
Adv. Funct. Mater. 28, 1800976 (2018)
Schulz, A., Stocco, A., Bethry, A., Lavigne, J.-P., Coudane, J. & Nottelet, B.

ABSTRACT
The possibility to impart surface properties to any polymeric substrate using a fast, reproducible and industrially friendly procedure, without the need for surface pre-treatment, is highly sought after. This is in particular true in the frame of antibacterial surfaces to hinder the threat of biofilm formation. In this study we demonstrate the potential of aryl-azide polymers for photo-functionalization and the importance of the polymer structure for an efficient grafting. The strategy is illustrated with a UV-reactive hydrophilic poly(2-oxazoline) based copolymer, which can be photografted onto any polymer substrate that contains carbon-hydrogen bonds to introduce antifouling properties. Through detailed characterization it is demonstrated that the controlled spatial distribution of the UV-reactive aryl-azide moieties within the poly(2-oxazline) structure, in the form of pseudo gradient copolymers, ensures higher grafting efficacy than other copolymer structures including block copolymers. Furthermore, it is found that the photografting results in a covalently bound layer, which is thermally stable and causes a significant anti-adherence effect and biofilm reduction against E. coli and S. epidermidis strains while remaining non-cytotoxic against mouse fibroblasts.
N-(2-Hydroxypropyl)methacrylamide-Based Linear, Diblock, and Starlike Polymer Drug Carriers: Advanced Process for Their Simple Production
This is custom heading element
Biomacromolecules 19, 4003–4013 (2018)
Koziolova, E., Kostka, L., Kotrchova, L., Subr, V., Konefal, R., Nottelet, B. & Etrych, T.
ABSTRACT
We developed a new simplified method for the synthesis of well-defined linear, diblock, or starlike N-(2-hydroxypropyl)methacrylamide (HPMA)-based polymer drug carriers using controlled reversible addition–fragmentation chain transfer polymerization. The prepared monodispersed polymers are after the drug attachment intended for enhanced anticancer therapy. This new approach significantly reduces the number of required synthetic steps and minimizes the consumption of organic solvents during the synthesis. As a result, highly defined linear, diblock, and starlike copolymers designed for pH-triggered drug activation/release in tumor tissue were formed in sufficient amounts for further physicochemical and biological studies. Within the synthesis, we also developed a new procedure for the selective deprotection of tert-butoxycarbonyl hydrazide and amine groups on hydrophilic HPMA copolymers, including the one-pot removal of polymer end groups. We studied and described in detail the kinetics and efficacy of the deprotection reaction. We believe the simplified synthetic approach facilitates the preparation of polymer conjugates bound by the pH-sensitive hydrazone bond and their application in tumor treatment.
A new bioabsorbable polymer film to prevent peritoneal adhesions validated in a post-surgical animal model
This is custom heading element
Allegre, L., Le Teuff, I., Leprince, S., Warembourg, S., Taillades, H., Garric, X., Letouzey, V. & Huberlant, S.
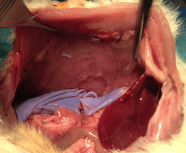
ABSTRACT
Background – Peritoneal adhesions are a serious surgical postoperative complication. The aim of this study is to investigate, in a rat model, the anti-adhesive effects of a bioabsorbable film of polymer combining polyethylene glycol and polylactic acid that can be easily applied during surgery.
Materials and Methods – Sixty three animals were randomized into five groups according to the anti- adhesion treatment: Hyalobarrier®, Seprafilm®, Polymer A (PA), Polymer B (PB), and control. The rats were euthanized on days 5 and 12 to evaluate the extent, severity and degree of adhesions and histopathological changes. Three animals were euthanized at day 2 in PA, PB and control groups to observe the in vivo elimination.
Results – Macroscopic adhesion formation was significantly lower in the PA group than in the control group at day 5 (median adhesion score 0±0 vs 9.6 ±0.5 p=0.002) and at day 12 (0±0 vs 7.3±4 p=0.02). Furthermore, median adhesion score at day 5 was significantly lower in the PA group than in the Seprafilm® group (0±0 vs 4.2± 3.9 p = 0.03). Residence time of PA seems longer than PB.
Conclusion – The PA bioabsorbable film seems efficient in preventing the formation of peritoneal adhesions
Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing
This is custom heading element
Acta Biomater. 76, 164–177 (2018)
Pinese, C., Lin, J., Milbreta, U., Li, M., Wang, Y., Leong, K. W. & Chew, S. Y.
ABSTRACT
A low toxicity and efficient delivery system is needed to deliver small interfering RNAs (siRNA) in vitro and in vivo. The use of mesoporous silica nanoparticles (MSN) is becoming increasingly common due to its biocompatibility, tunable pore size and customizable properties. However, bolus delivery of siRNA/MSN complexes remains suboptimal, especially when a sustained and long-term administration is required. Here, we utilized electrospun scaffolds for sustained delivery of siRNA/MSN-PEI through surface adsorption and nanofiber encapsulation. As a proof-of-concept, we targeted collagen type I expression to modulate fibrous capsule formation. Surface adsorption of siRNA/MSN-PEI provided sustained availability of siRNA for at least 30 days in vitro. As compared to conventional bolus delivery, such scaffold-mediated transfection provided more effective gene silencing (p < 0.05). On the contrary, a longer sustained release was attained (at least 5 months) when siRNA/MSN-PEI complexes were encapsulated within the electrospun fibers. In vivo subcutaneous implantation and biodistribution analysis of these scaffolds revealed that siRNA remained localized up to similar to 290 mu m from the implants. Finally, a fibrous capsule reduction of similar to 45.8% was observed after 4 weeks in vivo as compared to negative scrambled siRNA treatment. Taken together, these results demonstrate the efficacy of scaffold-mediated sustained delivery of siRNA/MSN-PEI for long-term non-viral gene silencing applications. Statement of Significance The bolus delivery of siRNA/mesoporous silica nanoparticles (MSN) complexes shows high efficiency to silence protein agonists of tumoral processes as cancer treatments. However, in tissue engineering area, scaffold mediated delivery is desired to achieve a local and sustained release of therapeutics. We showed the feasibility and the efficacy of siRNA/MSN delivered from electrospun scaffolds through surface adsorption and nanofiber encapsulation. We showed that this method enhances siRNA transfection efficiency and sustained targeted proteins silencing in vitro and in vivo. As a proof of concept, in this study, we targeted collagen type I expression to modulate fibrous capsule formation. However this platform can be applied to the release and transfection of siRNA or miRNA in cancer and tissue engineering applications.
Engineered nanomaterials and human health: Part 1. Preparation, functionalization and characterization (IUPAC Technical Report).
This is custom heading element
Pure Appl. Chem. 90, 1283–1324 (2018)
Gubala, V., Johnston, L. J., Liu, Z., Krug, H., Moore, C. J., Ober, C. K., Schwenk, M. & Vert, M.

ABSTRACT
Nanotechnology is a rapidly evolving field, as evidenced by the large number of publications on the synthesis, characterization, and biological/environmental effects of new nano-sized materials. The unique, size-dependent properties of nanomaterials have been exploited in a diverse range of applications and in many examples of nano-enabled consumer products. In this account we focus on Engineered Nanomaterials (ENM), a class of deliberately designed and constructed nano-sized materials. Due to the large volume of publications, we separated the preparation and characterisation of ENM from applications and toxicity into two interconnected documents. Part 1 summarizes nanomaterial terminology and provides an overview of the best practices for their preparation, surface functionalization, and analytical characterization. Part 2 (this issue, Pure Appl. Chem. 2018; 90(8): 1325–1356) focuses on ENM that are used in products that are expected to come in close contact with consumers. It reviews nanomaterials used in therapeutics, diagnostics, and consumer goods and summarizes current nanotoxicology challenges and the current state of nanomaterial regulation, providing insight on the growing public debate on whether the environmental and social costs of nanotechnology outweigh its potential benefits.
PLA scaffolds production from Thermally Induced Phase Separation: Effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide
This is custom heading element
Supercrit. Fluids 136, 123–135 (2018)
Gay, S., Lefebvre, G., Bonnin, M., Nottelet, B., Boury, F., Gibaud, A. & Calvignac, B.

ABSTRACT
In this work, a relatively large scale of PLA scaffolds was produced using thermally induced phase separation (TIPS) combined with a supercritical carbon dioxide (SC-CO2) drying step as a green alternative. For the TIPS step, the phase separation of PLA and 1,4-dioxane solvent was controlled by adjusting the process conditions such as the polymer concentration and molecular weight, the 1,4-dioxane solvent power and the cooling conditions. The scaffolds morphology was analyzed by scanning electron microscopy. Their structural and mechanical properties were correlated together with the possibility to tune them by controlling the process conditions. An environmental analysis using the Life Cycle Assessment (LCA) methodology confirmed a reduction of at least 50% of the environmental impact of the whole process using the SC-CO2 drying compared to the traditional freeze-drying technology. This work is the first known attempt to conduct the LCA methodology on TIPS process for the PLA scaffolds production.
Site-specific grafting on titanium surfaces with hybrid temporin antibacterial peptides.
This is custom heading element
Mat. Chem. B 6, 1782–1790 (2018)
Masurier, N., Tissot, J.-B., Boukhriss, D., Jebors, S., Pinese, C., Verdie, P., Amblard, M., Mehdi, A., Martinez, J., Humblot, V. & Subra, G.
ABSTRACT
Relying on a membrane-disturbing mechanism of action and not on any intracellular target, antimicrobial peptides (AMP) are attractive compounds to be grafted on the surface of implantable materials such as silicone catheters or titanium surgical implants. AMP sequences often display numerous reactive functions (e.g. amine, carboxylic acid) on their side chains and straightforward conjugation chemistries could lead to uncontrolled covalent grafting, random orientation, and non-homogenous density. To achieve an easy and site specific covalent attachment of unprotected peptides on titanium surfaces, we designed hybrid silylated biomolecules based on the temporin-SHa amphipathic helical antimicrobial sequence. With the grafting reaction being chemoselective, we designed five analogues displaying the silane anchoring function at the N-ter, C-ter or at different positions inside the sequence to get an accurate control of the orientation. Grafting density calculations were performed by XPS and the influence of the orientation of the peptide on the surface was clearly demonstrated by the measure of antimicrobial activity. Temporin amphipathic helices are described to permeabilize the bacterial membrane by interacting in a parallel orientation with it. Our results move in the direction of this mechanism as the selective grafting of hybrid temporin 2 through a lysine placed at the center of the peptide sequence, resulted in better biofilm growth inhibition of E. coli and S. epidermis than substrates in which temporins were grafted via their C- or N-terminus.
Prebiotic macromolecules and today’s biomacromolecules in the light of polymerology
This is custom heading element
Eur. Polym. J. 100, 25–36 (2018)
Vert, M.
ABSTRACT
The appearance of life and thus of biomacromolecules on Earth is related to the presence of small organic molecules, polymerized under prebiotic environmental conditions. However, none of proteins, polynucleotides or polysaccharides taken individually is a true biomacromolecule, because biomacromolecules result from mutual involvements in complex biological processes. Logically, the biomacromolecules involved in the first living cells were first macromolecules formed under early Earth environmental conditions. The appearance of such living cells required macromolecules with fundamental features like controlled synthesis and structures, compatibility with aqueous media, chirality, memory, replication, and recycling that had to respect corresponding fundamentals of polymer science referred to as polymerology. After a recall of relevant basics of chemical reactions, the prebiotic appearance of macromolecules is critically compared with today’s polymer science including chirality, stability, degradation, and recycling. Instead of providing arguments in favor of consistent routes to the first biomacromolecules, the reference to polymerology emphasizes obstacles that complement those occasionally found in the origin of life literature. In front of this conclusion, the discussion is extended to the other ends of these routes, i.e. today’s biomacromolecules. The comparison with polymerology emphasizes the pertinence of the natural choices that led to the outstanding smartness of biomacromolecules. Polymerology is still in its cradle after less than a century of existence. For the future, it is suggested to pay increasing attention to chiral, multimeric multifunctional macromolecules to enrich the population of smart polymers, to solve the problem of plastic pollution, and, maybe, to enlighten the mystery of biomacromolecules emergence under unfavorable conditions.
Engineered nanomaterials and human health: Part 2. Applications and nanotoxicology (IUPAC Technical Report)
This is custom heading element
Pure Appl. Chem. 90, 1325–1356 (2018)
Gubala, V., Johnston, L. J., Krug, H. F., Moore, C. J., Ober, C. K., Schwenk, M. & Vert, M.

ABSTRACT
Research on engineered nanomaterials (ENM) has progressed rapidly from the very early stages of studying their unique, size-dependent physicochemical properties and commercial exploration to the development of products that influence our everyday lives. We have previously reviewed various methods for synthesis, surface functionalization, and analytical characterization of ENM in a publication titled ‘Engineered Nanomaterials: Preparation, Functionalization and Characterization’. In this second, inter-linked document, we first provide an overview of important applications of ENM in products relevant to human healthcare and consumer goods, such as food, textiles, and cosmetics. We then highlight the challenges for the design and development of new ENM for bio-applications, particularly in the rapidly developing nanomedicine sector. The second part of this document is dedicated to nanotoxicology studies of ENM in consumer products. We describe the various biological targets where toxicity may occur, summarize the four nanotoxicology principles, and discuss the need for careful consideration of the biodistribution, degradation, and elimination routes of nanosized materials before they can be safely used. Finally, we review expert opinions on the risk, regulation, and ethical aspects of using engineered nanomaterials in applications that may have direct or indirect impact on human health or our environment.
Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters
This is custom heading element
Environ. Pollut. 236, 807–816 (2018)
Dussud, C., Meistertzheim, A. L., Conan, P., Pujo-Pay, M., George, M., Fabre, P., Coudane, J., Higgs, P., Elineau, A., Pedrotti, M. L., Gorsky, G. & Ghiglione, J. F.

ABSTRACT
Research on engineered nanomaterials (ENM) has progressed rapidly from the very early stages of studying their unique, size-dependent physicochemical properties and commercial exploration to the development of products that influence our everyday lives. We have previously reviewed various methods for synthesis, surface functionalization, and analytical characterization of ENM in a publication titled ‘Engineered Nanomaterials: Preparation, Functionalization and Characterization’. In this second, inter-linked document, we first provide an overview of important applications of ENM in products relevant to human healthcare and consumer goods, such as food, textiles, and cosmetics. We then highlight the challenges for the design and development of new ENM for bio-applications, particularly in the rapidly developing nanomedicine sector. The second part of this document is dedicated to nanotoxicology studies of ENM in consumer products. We describe the various biological targets where toxicity may occur, summarize the four nanotoxicology principles, and discuss the need for careful consideration of the biodistribution, degradation, and elimination routes of nanosized materials before they can be safely used. Finally, we review expert opinions on the risk, regulation, and ethical aspects of using engineered nanomaterials in applications that may have direct or indirect impact on human health or our environment.
A method to slow down the ionization-dependent release of risperidone loaded in a thermoresponsive poly(N-acryloyl glycinamide) hydrogel
This is custom heading element
Drug Deliv. Transl. Res. 7, 460–464 (2017)
Boustta, M. & Vert, M.

ABSTRACT
Poly(N-acryloyl glycinamide) polymers are soluble in hot aqueous media that gel rapidly on cooling. This gelatin-like behavior was previously compared with drug delivery requirements. Slow releases were demonstrated in vitro using different model molecules and macromolecules and in vivo using methylene blue. Risperidone is a weak basic drug sparingly soluble in water frequently used to treat patients suffering of schizophrenia. A standard risperidone-poly(N-acryloyl glycinamide) hydrogel formulation was selected from which the drug was allowed to release comparatively in buffered and non-buffered isotonic media at 37 °C under pseudo sink conditions. Linear release was observed in pH = 7.4 phosphate buffer whereas in buffer-free 0.15 M NaCl, the release was initially faster than in the buffer but became rapidly slower as the pH increased from 6.8 to 8.2. These features were related to the ionization-dependent solubility of risperidone. In order to minimize the ionization and thus the solubility of the drug inside the hydrogel despite outside buffering at 7.4, Mg(OH2), a sparingly soluble mineral base, was added to the standard formulation. This addition resulted in a c.a. threefold increase of the zero-order release duration. The method should be applicable to other sparingly soluble weakly basic drugs.
In vivo evaluation of hybrid patches composed of PLA based copolymers and collagen/chondroitin sulfate for ligament tissue regeneration: Hybrid Patches for Ligament Reconstruction
This is custom heading element
J. Biomed. Mater. Res. B 105, 1778–1788 (2017)
Pinese, C., Gagnieu, C., Nottelet, B., Rondot-Couzin, C., Hunger, S., Coudane, J. & Garric, X.
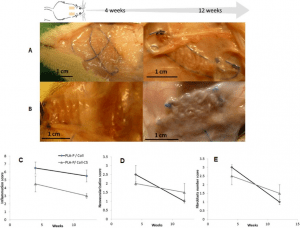
ABSTRACT
Biomaterials for soft tissues regeneration should exhibit sufficient mechanical strength, demonstrating a mechanical behavior similar to natural tissues and should also promote tissues ingrowth. This study was aimed at developing new hybrid patches for ligament tissue regeneration by synergistic incorporation of a knitted structure of degradable polymer fibers to provide mechanical strength and of a biomimetic matrix to help injured tissues regeneration. PLA‐ Pluronic® (PLA‐P) and PLA‐Tetronic® (PLA‐T) new copolymers were shaped as knitted patches and were associated with collagen I (Coll) and collagen I/chondroitine‐sulfate (Coll CS) 3‐dimensional matrices. In vitro study using ligamentocytes showed the beneficial effects of CS on ligamentocytes proliferation. Hybrid patches were then subcutaneously implanted in rats for 4 and 12 weeks. Despite degradation, patches retained strength to answer the mechanical physiological needs. Tissue integration capacity was assessed with histological studies. We showed that copolymers, associated with collagen and chondroitin sulfate sponge, exhibited very good tissue integration and allowed neotissue synthesis after 12 weeks in vivo. To conclude, PLA‐P/CollCS and PLA‐T/CollCS hybrid patches in terms of structure and composition give good hopes for tendon and ligament regeneration.
Robust & thermosensitive poly(ethylene glycol)-poly(e-caprolactone) star block copolymer hydrogels
This is custom heading element
Polym. Degrad. Stabil. 137, 173–183 (2017)
Buwalda, S. J., Nottelet, B. & Coudane, J.
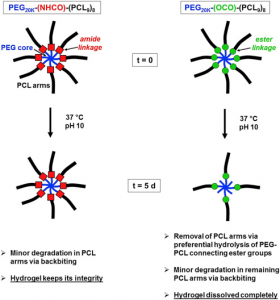
ABSTRACT
Novel 8-armed poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) star block copolymers, possessing an amide or an ester group between the PEG core and the PCL arms (PEG20K-(NHCO)-(PCL9)8 and PEG20K-(OCO)-(PCL9)8), are synthesized by ring opening polymerization of ε-caprolactone in toluene at 110 °C initiated by 8-armed star PEG20K-(NH2)8 and PEG20K-(OH)8, respectively. Compared to linear triblock copolymers with a similar hydrophilic/hydrophobic balance and molecular weight, star block copolymers show better aqueous solubility and yield more homogeneous and transparent hydrogels. PEG20K-(NHCO)-(PCL9)8 hydrogels exhibit a significantly higher storage modulus and in vitro stability in comparison with PEG20K-(OCO)-(PCL9)8 hydrogels of similar concentration and molecular weight. 1H NMR analysis of degrading hydrogel samples clearly demonstrates different degradation mechanisms for the ester and amide type star block copolymers. Their robust mechanical properties, the possibility to be formed in situ and their excellent resistance against hydrolytic degradation make these PEG-PCL star block copolymer hydrogels, especially those based on PEG20K-(NHCO)-(PCL9)8, appealing for various biomedical applications.
A preclinical evaluation of polypropylene/polylacticacid hybrid meshes for fascial defect repair using a rat abdominal hernia model
This is custom heading element
Ulrich, D., Le Teuff, I., Huberlant, S., Carteron, P., Letouzey, V. & de Tayrac, R.
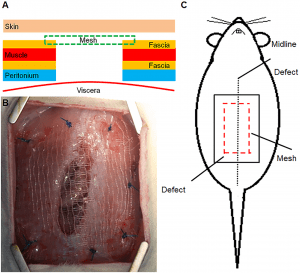
ABSTRACT
Objectives
Synthetic mesh surgery for both abdominal and urogenital hernia repair is often unsatisfactory in the long-term due to postoperative complications. We hypothesized that a semi-degradable mesh hybrid may provide more appropriate biocompatibility with comparable mechanical properties. The aim was to compare its in vivo biocompatibility with a commercial polypropylene (PP) mesh.
Methods
72 rats were randomly allocated to either our new composite mesh (monofilament PP mesh knitted with polylactic-acid-fibers (PLA)) or to a commercially available PP mesh that was used as a control. 15, 90, and 180 days after implantation into the rat abdomen mesh tissue complexes were analysed for erosion, contraction, foreign body reaction, tissue integration and biomechanical properties.
Results
No differences were seen in regard to clinical parameters including erosion, contraction or infection rates between the two groups. Biomechanical properties including breaking load, stiffness and deformation did not show any significant differences between the different materials at any timepoint. Macrophage staining did not reveal any significant differences between the two groups or between timepoints either. In regard to collagen I there was significantly less collagen I in the PP group compared to the PP/ PLA group at day 180. Collagen III did not show any significant differences at any timepoint between the two groups.
Conclusion
A PP/PLA hybrid mesh, leaving a low amount of PP after PLA degradation seems to have comparable biomechanical properties like PP at 180 days due to enhanced collagen production without significant differences in erosion, contraction, herniation or infection rates.
Bioactive peptides grafted silicone dressings: A simple and specific method
This is custom heading element
Materials Today Chemistry 4, 73–83 (2017)
Pinese, C., Jebors, S., Stoebner, P. E., Humblot, V., Verdié, P., Causse, L., Garric, X., Taillades, H., Martinez, J., Mehdi, A. & Subra, G
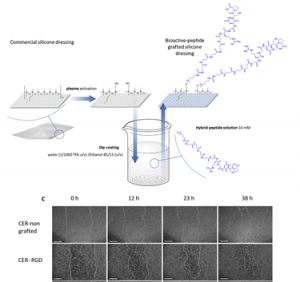
ABSTRACT
The need for bioactive dressings increases with the population aging and the prevalence of chronic diseases. In contrast, there are very few dressings on the market which are designed to display a chosen bioactivity. In this context, we investigated the surface-functionalization of silicone wound dressing with bioactive peptides. One of the challenges was to avoid multistep grafting reactions involving catalysts, solvents or toxic reagents, which are not suitable for the fabrication of medical devices at an industrial scale. In the other hand, a covalent bonding was necessary to avoid the loss of the biological effect by progressive removal of the peptide in biological fluids generated by the wound. To solve these limitations, we developed a strategy allowing an easy and direct functionalization of silicone. This strategy relies on hybrid silylated bioactive peptides, which chemoselectively react with plasma-activated silicone surfaces. We synthesized three hybrid peptides with wound healing properties, which were grafted on commercially available silicone dressings Cerederm® and Mepitel®. Grafted dressings were evaluated in vitro and enabled a quicker scare recovery and extracellular matrix deposition with human dermal fibroblasts. These results were confirmed by in vivo studies showing an enhanced wound-healing of the pig skin. By this simple method, we transformed inert dressing into bioactive dressing which showed properties of wound healing.
Nanoprecipitated catestatin released from pharmacologically active microcarriers (PAMs) exerts pro-survival effects on MSC
This is custom heading element
Angotti, C., Venier-Julienne, M. C., Penna, C., Femmino, S., Sindji, L., Paniagua, C., Montero-Menei, C. N. & Pagliaro, P
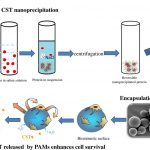
ABSTRACT
Catestatin (CST), a fragment of Chromogranin-A, exerts angiogenic, arteriogenic, vasculogenic and cardioprotective effects. CST is a very promising agent for revascularization purposes, in “NOOPTION” patients. However, peptides have a very short half-life after administration and must be conveniently protected. Fibronectin-coated pharmacologically active microcarriers (FN-PAM), are biodegradable and biocompatible polymeric microspheres that can convey mesenchymal stem cell (MSCs) and therapeutic proteins delivered in a prolonged manner. In this study, we first evaluated whether a small peptide such as CST could be nanoprecipitated and incorporated within FN-PAMs. Subsequently, whether CST may be released in a prolonged manner by functionalized FN-PAMs (FN-PAM-CST). Finally, we assessed the effect of CST released by FN-PAM-CST on the survival of MSCs under stress conditions of hypoxia-reoxygenation. An experimental design, modifying three key parameters (ionic strength, mixing and centrifugation time) of protein nanoprecipitation, was used to define the optimum condition for CST. An optimal nanoprecipitation yield of 76% was obtained allowing encapsulation of solid CST within FN-PAM-CST, which released CST in a prolonged manner. In vitro, MSCs adhered to FN-PAMs, and the controlled release of CST from FN-PAM-CST greatly limited hypoxic MSC-death and enhanced MSC-survival in post-hypoxic environment. These results suggest that FN-PAM-CST are promising tools for cell-therapy.
Rolled knitted scaffolds based on PLA-pluronic copolymers for anterior cruciate ligament reinforcement: A step by step conception
This is custom heading element
J. Biomed. Mater. Res. B 105, 735–743 (2017)
Pinese, C., Gagnieu, C., Nottelet, B., Rondot-Couzin, C., Hunger, S., Coudane, J. & Garric, X.
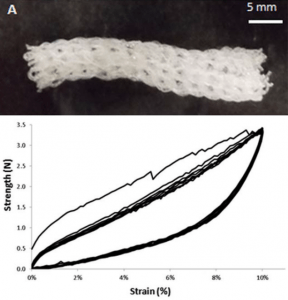
ABSTRACT
The aim of this study was to prepare a new knitted scaffold from PLA‐Pluronic block copolymers for anterior cruciate ligament reconstruction. The impact of sterilization methods (beta‐ray and gamma‐ray sterilization) on copolymers was first evaluated in order to take into account the possible damages due to the sterilization process. Beta‐ray radiation did not significantly change mechanical properties in contrast to gamma‐ray sterilization. It was shown that ACL cells proliferate onto these copolymers, demonstrating their cytocompatibility. Thirdly, in order to study the influence of shaping on mechanical properties, several shapes were created with copolymers yarns: braids, ropes and linear or rolled knitted scaffolds. The rolled knitted scaffold presented interesting mechanical characteristics, similar to native anterior cruciate ligament (ACL) with a 67 MPa Young’s Modulus and a stress at failure of 22.5 MPa. These findings suggest that this three dimensional rolled knitted scaffold meet the mechanical properties of ligament tissues and could be suitable as a scaffold for ligament reconstruction.
Sol-gel synthesis of collagen-inspired peptide hydrogel
This is custom heading element
Echalier, C., Jebors, S., Laconde, G., Brunel, L., Verdie, P., Causse, L., Bethry, A., Legrand, B., Van Den Berghe, H., Garric, X., Noel, D., Martinez, J., Mehdi, A. & Subra, G.
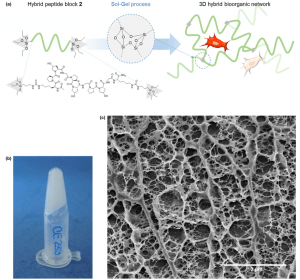
ABSTRACT
Conceiving biomaterials able to mimic the specific environments of extracellular matrices are a prerequisite for tissue engineering applications. Numerous types of polymers (PEG, PLA, etc.) have been used for the design of biocompatible scaffolds, but they are still less efficient than natural biopolymers such as collagen extracts. Chemically modified and loaded with different bioactive factors, biopolymers afford an environment favourable to cell proliferation and differentiation. Unfortunately, they present several drawbacks, such as weak batch-to-batch reproducibility, potential immunogenicity and high cost of production. Herein we propose a fully synthetic covalent hydrogel obtained by sol–gel polymerization of a silylated peptide. We selected a short and low molecular building-block derived from the consensus collagen sequence [Pro-Hyp-Gly]. Interestingly, the sol–gel process occurs in physiological buffer, enabling the embedment of stem cells. This collagen-inspired hydrogel provides a cell-friendly environment comparable to natural collagen substrates, demonstrating its potency as a biomimetic scaffold.
Comparison between protein repulsions by diblock PLA-PEO and albumin nanocoatings using OWLS
This is custom heading element
J. Biomater. Sci.-Polym. Ed. 28, 177–193 (2017)
Leclercq, L. & Vert, M

ABSTRACT
A previous investigation suggested that a surface bearing a rinsing-resistant depot (nanocoating) of albumin is more protein-repulsive than the same surface physically pegylated by a poly(D,L-lactic acid)-poly(ethylene oxide) diblock copolymer. To complement the study, Optical Waveguide Lightmode Spectroscopy was used to compare the mass and the thickness of protein depots from different systems, namely albumin alone at different concentrations, a mixture of albumin + fibrinogen + γ-globulin at their physiological concentrations, and sheep serum. The same standard OWLS protocol was applied to compare data for bare sensor chips, for chips covered by an albumin nanocoating, and for chips physically pegylated using poly(D,L-lactic acid)-poly(ethylene oxide) diblock copolymers with different compositions and block lengths. The strategy and the conditions being rather different from those generally used to study pegylation-related antifouling properties; the literature was first reviewed critically. Then full coverage of sensor chips by albumin was demonstrated. The comparative study confirmed that albumin was more protein-repulsive than any of the diblock copolymers, irrespective of the protein system. Furthermore, it was found that pegylated surfaces were albumin-repulsive only when the concentration of the protein solution flowing over the surface was very low (0.1 g/L). It was not possible to correlate the copolymer data to PEO chain density, chain length and existence of brush. The in vitro repulsive activity of albumin was not affected by drying and rehydration, a feature of interest for storage of albumin-coated surfaces. All these observations confirmed our preliminary findings and showed that considering model proteins individually or in mixtures at concentrations far from physiological concentrations are not suitable to reflect the reality of full blood-surface interactions.
Modular bioink for 3D printing of biocompatible hydrogels: sol–gel polymerization of hybrid peptides and polymers
This is custom heading element
RSC Advances 7, 12231–12235 (2017)
Echalier, C., Levato, R., Mateos-Timoneda, M. A., Castaño, O., Déjean, S., Garric, X., Pinese, C., Noël, D., Engel, E., Martinez, J., Mehdi, A. & Subra, G
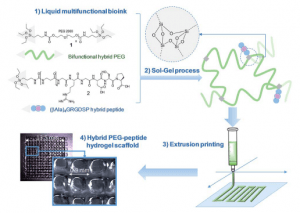
ABSTRACT
An unprecedented generic system allowing the 3D printing of peptide-functionalized hydrogels by soft sol –gel inorganic polymerization is presented. Hybrid silylated inorganic/bioorganic blocks are mixed in biological buffer in an appropriate ratio, to yield a multicomponent bioink that can be printed as a hydrogel without using any photochemical or organic reagent. Hydrolysis and condensation of the silylated precursors occur during the printing process and result in a covalent network in which molecules are linked through siloxane bonds. The viscosity of the colloidal solution used as bioink was monitored in order to set up the optimal conditions for extrusion printing. Grid-patterned hydrogel scaffolds containing a hybrid integrin ligand were printed using a pressure-driven rapid prototyping machine. Finally, they were seeded with mesenchymal stem cells, demonstrating their suitability for cell culture. The versatility of the sol – gel process and its biocompatibility makes this approach highly promising for the preparation of tailor-made cell-laden scaffolds.
Pharmacologically active microcarriers delivering BDNF within a hydrogel: Novel strategy for human bone marrow-derived stem cells neural neuronal differentiation guidance and therapeutic secretome enhancement
This is custom heading element
Acta Biomater. 49, 167–180 (2017)
Kandalam, S., Sindji, L., Delcroix, G. J.-R., Violet, F., Garric, X., Andre, E. M., Schiller, P. C., Venier-Julienne, M.-C., des Rieux, A., Guicheux, J. & Montero-Menei, C. N.
ABSTRACT
Stem cells combined with biodegradable injectable scaffolds releasing growth factors hold great promises in regenerative medicine, particularly in the treatment of neurological disorders. We here integrated human marrow-isolated adult multilineage-inducible (MIAMI) stem cells and pharmacologically active microcarriers (PAMs) into an injectable non-toxic silanized-hydroxypropyl methylcellulose (Si-HPMC) hydrogel. The goal is to obtain an injectable non-toxic cell and growth factor delivery device. It should direct the survival and/or neuronal differentiation of the grafted cells, to safely transplant them in the central nervous system, and enhance their tissue repair properties. A model protein was used to optimize the nanoprecipitation conditions of the neuroprotective brain-derived neurotrophic factor (BDNF). BDNF nanoprecipitate was encapsulated in fibronectin-coated (FN) PAMs and the in vitro release profile evaluated. It showed a prolonged, bi-phasic, release of bioactive BDNF, without burst effect. We demonstrated that PAMs and the Si-HPMC hydrogel increased the expression of neural/neuronal differentiation markers of MIAMI cells after 1 week. Moreover, the 3D environment (PAMs or hydrogel) increased MIAMI cells secretion of growth factors (b-NGF, SCF, HGF, LIF, P1GF-1, SDF-1 alpha, VEGF-A & D) and chemokines (MIP-la & RANTES, IL-8). These results show that PAMs delivering BDNF combined with Si-HPMC hydrogel represent a useful novel local delivery tool in the context of neurological disorders. It not only provides neuroprotective BDNF but also bone marrow-derived stem cells that benefit from that environment by displaying neural commitment and an improved neuroprotective/reparative secretome. It provides preliminary evidence of a promising pro-angiogenic, neuroprotective and axonal growth-promoting device for the nervous system. Statement of Significance Combinatorial tissue engineering strategies for the central nervous system are scarce. We developed and characterized a novel injectable non-toxic stem cell and protein delivery system providing regenerative cues for central nervous system disorders. BDNF, a neurotrophic factor with a wide-range effect, was nanoprecipitated to maintain its structure and released in a sustained manner from novel polymeric microcarriers. The combinatorial 3D support, provided by fibronectin-microcarriers and the hydrogel, to the mesenchymal stem cells guided the cells towards a neuronal differentiation and enhanced their tissue repair properties by promoting growth factors and cytokine secretion. The long-term release of physiological doses of bioactive BDNF, combined to the enhanced secretion of tissue repair factors from the stem cells, constitute a promising therapeutic approach.
Redox Reducible and Hydrolytically Degradable PEG-PLA Elastomers as Biomaterial for Temporary Drug-Eluting Medical Devices.
This is custom heading element
Macromolecular Bioscience 16, 1792–1802 (2016)
Rupnik, S., Buwalda, S., Dejean, S., Bethry, A., Garric, X., Coudane, J. & Nottelet, B

ABSTRACT
With the aim to develop biomaterials for temporary medical devices, a series of novel reducible and/or degradable elastomers has been prepared from PLA‐b‐PEG‐b‐PLA copolymers photo‐crosslinked with diallyl sulfide or pentaerythritol tetrakis(3‐mercaptopropionate). Thermal and mechanical properties, including elastic limit and Young modulus, are assessed. Degradation is then evaluated under standard hydrolytic conditions. Reducibility of a selected elastomer is then illustrated using 2‐mercaptoethanol or glutathione as reducing agents. The redox‐sensitivity of the selected elastomer and the possibility to modulate the degradability are shown. Considering drug‐eluting elastomeric devices applications, anti‐inflammatory drug ibuprofen loading is illustrated with the two simplest elastomer formulations. A rapid or slow linear release is observed as a function of the low or high molecular weight of the triblock pre‐polymers. Finally, the cytocompatibility of the degradable elastomers is assessed with regard to their potential to favor or inhibit L929 murine fibroblasts proliferation as a function of the hydrophilicity/hydrophobicity of the triblock copolymers.
PCL-PEG graft copolymers with tunable amphiphilicity as efficient drug delivery systems
This is custom heading element
Mat. Chem. B 4, 6228–6239 (2016)
Al Samad, A., Bethry, A., Koziolova, E., Netopilik, M., Etrych, T., Bakkour, Y., Coudane, J., El Omar, F. & Nottelet, B.
ABSTRACT
The development of flexible drug delivery systems that can be tuned as a function of the drug to be delivered and of the target disease is crucial in modern medicine. For this aim, novel amphiphilic poly(ε-caprolactone)-g-poly(ethylene glycol) (PCL-g-PEG) copolymers with well-controlled design were synthesized by thiol–yne photochemistry. The grafting density and the copolymer amphiphilicity were easily controlled via the reaction parameters: concentration, reaction time, PEG length and the molar ratio between PCL and PEG or the photoinitiator in the reaction mixture. The self-assembling behavior of the copolymers was studied and a correlation between the composition of PCL-g-PEG and the nanoaggregate diameter sizes (28 to 73 nm) and critical aggregation concentrations (1.1 to 4.3 mg L−1) was found. The influence of copolymer amphiphilicity on the drug loading was evaluated with various drugs including anticancer drugs (paclitaxel, ABT-199), drugs to overcome multidrug resistance in cancer cells (curcumin, elacridar), an anti-inflammatory drug (dexamethasone) and an antibacterial drug (clofazimine). Finally, the influence of amphiphilicity on curcumin release and toxicity towards MCF-7 cancer cell lines was studied. The impact of the grafting density, PEG length and the overall EG/CL ratio is discussed in detail. Curcumin loaded PCL-g-PEG with lower EG/CL ratios and shorter PEG chains showed higher toxicity compared to their more hydrophilic counterparts.
A new water-soluble polycarbobetaine showing high selectivity toward copper
This is custom heading element
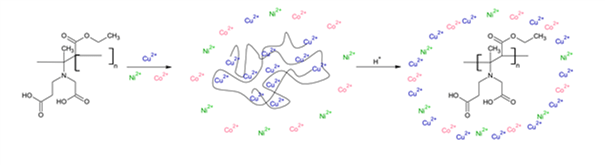
ABSTRACT
In this study, betaine-type polyampholytes (polycarbobetaines, PCBets) are investigated as complexing agents of metal ions. Because PCBets are pH-sensitive due to the presence of amine and carboxylic acid groups, the complexation of copper was monitored at various pH values (from 3 to 6) by UV–vis spectroscopy and/or electrochemical measurements. The results obtained for the complexation of copper were greatly enhanced as the pH increased and reached 264 ± 11 mg g1 at pH = 6. This maximum adsorption capacity rivals the recent results obtained for molecules of environmental interest, such as chitosan. The selectivity of the target PCBets for copper was indicated in the presence of nickel and/or cobalt and partially accounts for the interest in this material and demonstrated the relevance of using
PCBet in environmental applications (such as metal recovery from wastes/wastewaters or environmental sensors). The efficiency of copper adsorption was maintained over 5 cycles of PCBet reuse (91 ± 4%).
MRI-visible polymer based on poly(methyl methacrylate) for imaging applications
This is custom heading element
Younis, M., Darcos, V., Paniagua, C., Ronjat, P., Lemaire, L., Nottelet, B., Garric, X., Bakkour, Y., El Nakat, J. H. & Coudane, J.

ABSTRACT
Macromolecular contrast agents are very attractive to afford efficient magnetic resonance imaging (MRI) visualization of implantable medical devices. In this work, we report on the grafting of a Gd-based DTPA contrast agent onto a poly(methyl methacrylate) derivative backbone by combining free radical polymerization and copper-catalyzed azide-alkyne cycloaddition (CuAAC). Using free radical polymerization, poly(methyl methacrylate-co-propargyl methacrylate) copolymers were prepared with a control of the ratio in propargyl methacrylate monomer units. The synthesis of a new azido mono-functionalized DTPA ligand was also reported and characterized by 1H NMR and mass spectroscopy. After complexation with gadolinium, this ligand has been grafted on the polymer backbone by click chemistry reaction. The obtained macromolecular contrast agent was then coated on a polypropylene mesh using the airbrushing technique and the mesh was assessed for MRI visualization at 7 teslas. The polymeric contrast agent was also tested for cytocompatibility and stability to assess its suitability for biomedical applications.
Simple and Specific Grafting of Antibacterial Peptides on Silicone Catheters
This is custom heading element
Advanced Healthcare Materials 5, 3067–3073 (2016)
Pinese, C., Jebors, S., Echalier, C., Licznar-Fajardo, P., Garric, X., Humblot, V., Calers, C., Martinez, J., Mehdi, A. & Subra, G.
ABSTRACT
To fight against nosocomial infection initiated by colonization of medical devices, a strategy enabling the direct and fast functionalization of silicone surfaces is proposed. This strategy proceeds in a site-specific way using original hybrid silylated antibacterial peptides. This safe and up-scalable method guarantees a covalent and robust immobilization with the correct orientation of the bioactive moiety. Importantly it also avoids multi-step chemical modifications of the surface or multi-layer polymer coatings. As proof of concept, antibacterial silicone catheter has been prepared whose immediate and long-term efficiency is superior by comparison to similar silver-embedded materials.
A non-linear viscoelastic model to describe the mechanical behavior’s evolution of biodegradable polymers during hydrolytic degradation
This is custom heading element
Polymer Degradation and Stability 131, 145–156 (2016)
Breche, Q., Chagnon, G., Machado, G., Nottelet, B., Garric, X., Girard, E. & Favier, D.
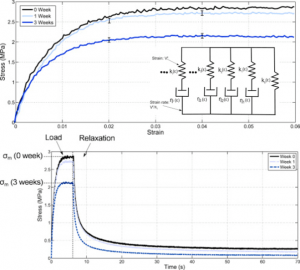
ABSTRACT
The biodegradable triblock copolymer PLA-b-PEG-b-PLA presents, in its initial state, a non-linear viscoelastic behavior. Its mechanical properties evolves during the in vitro degradation process. Tensile and relaxation tests are performed at 2%, 4% and 6% of load strain for different degradation steps. In order to describe the behavior of the polymer during degradation, an adaptive quasi-linear viscoelastic model is considered. In a first step, the model calibrated on the non-degraded state, perfectly fits the load and relaxation curves for every strain. Then, based on considerations about the preservation of the normalized relaxation curves over degradation time, the adaptive quasi-linear viscoelastic model is adapted to degradation. A degradation parameter that drives the mechanical degradation kinetics is deduced for every tested degradation states. A physically motivated model is finally used to describe the degradation parameter at every degradation step. The whole constitutive model is very accurate to fit the mechanical curves at every strain during degradation.
Mechanical behaviour׳s evolution of a PLA- b -PEG- b -PLA triblock copolymer during hydrolytic degradation.
This is custom heading element
Journal of the Mechanical Behavior of Biomedical Materials 60, 288–300 (2016)
Breche, Q., Chagnon, G., Machado, G., Girard, E., Nottelet, B., Garric, X. & Favier, D.
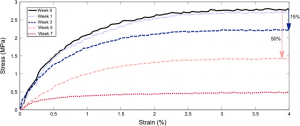
ABSTRACT
PLA-b-PEG-b-PLA is a biodegradable triblock copolymer that presents both the mechanical properties of PLA and the hydrophilicity of PEG. In this paper, physical and mechanical properties of PLA-b-PEG-b-PLA are studied during in vitro degradation. The degradation process leads to a mass loss, a decrease of number average molecular weight and an increase of dispersity index. Mechanical experiments are made in a specific experimental set-up designed to create an environment close to in vivo conditions. The viscoelastic behaviour of the material is studied during the degradation. Finally, the mechanical behaviour is modelled with a linear viscoelastic model. A degradation variable is defined and included in the model to describe the hydrolytic degradation. This variable is linked to physical parameters of the macromolecular polymer network. The model allows us to describe weak deformations but become less accurate for larger deformations. The abilities and limits of the model are discussed.
Formulation, physicochemical characterization and stability study of lithium-loaded microemulsion system
This is custom heading element
Int. J. Pharm. 502, 117–124 (2016)
Mouri, A., Legrand, P., El Ghzaoui, A., Dorandeu, C., Maurel, J. C. & Devoisselle, J.-M.
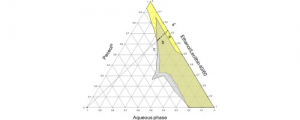
ABSTRACT
Lithium biocompatible microemulsion based on Peceol®, lecithin, ethanol and water was studied in attempt to identify the optimal compositions in term of drug content, physicochemical properties and stability. Lithium solubilization in microemulsion was found to be compatible with a drug-surfactant binding model. Lithium ions were predominantly solubilized within lecithin head group altering significantly the interfacial properties of the system. Pseudo-ternary phase diagrams of drug free and drug loaded microemulsions were built at constant ethanol/lecithin weight ratio (40/60). Lithium loaded microemulsion has totally disappeared in the Peceol® rich part of phase diagram; critical fractions of lecithin and ethanol were required for the formation of stable microemulsion. The effect of lithium concentration on the properties and physical stability of microemulsions were studied using microscopy, Karl Fischer titrations, rheology analyses, conductivity measurements and centrifugation tests. The investigated microemulsions were found to be stable under accelerated storage conditions. The systems exhibited low viscosity and behaved as Newtonian fluid and no structural transition was shown.
Easy Synthesis of Tunable Hybrid Bioactive Hydrogels
This is custom heading element
Chemistry of Materials 28, 1261–1265 (2016)
Echalier, C., Pinese, C., Garric, X., Van Den Berghe, H., Jumas Bilak, E., Martinez, J., Mehdi, A. & Subra, G.
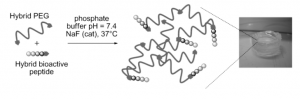
ABSTRACT
This work presented an easy and original method to prepare PEG-based hydrogels by the sol − gel process at physiological temperature and pH. We demonstrated through two examples that bioactive peptides can be covalently incorporated into gels and can confer their biological properties to the gels. Such gels could find applications in tissue engineering or in controlling postsurgical adhesions. In addition, this one-pot method to prepare biocompatible and bioactive hydrogels is highly adaptable. Indeed, gel properties can be finely tuned by mixing different building blocks to address a specific need for healthcare.
Self-assembly of well-defined triblock copolymers based on poly(lactic acid) and poly(oligo(ethylene glycol) methyl ether methacrylate) prepared by ATRP
This is custom heading element
RSC Adv. 6, 53370–53377 (2016)
Coumes, F., Beaute, L., Domurado, D., Li, S., Lecommandoux, S., Coudane, J. & Darcos, V.
ABSTRACT
Self-assembly of a series of amphiphilic poly(oligo(ethylene glycol) methyl ether methacrylate)-block-poly(lactic acid)-block-poly(oligo(ethylene glycol) methyl ether methacrylate) (P(OEGMA)-b-PLLA-b-P(OEGMA)) copolymers was investigated. The copolymers were synthesized by a combination of ring-opening polymerization (ROP) of L-lactide and atom transfer radical polymerization (ATRP) of oligo ethylene glycol methyl ether methacrylate (OEGMA). The resulting brush-like triblock copolymers were characterized by 1H NMR and size exclusion chromatography. Self-assembly behavior of the copolymers in deionized water was investigated by fluorescence spectroscopy, dynamic light scattering (DLS), and transmission electron microscopy (TEM). The critical aggregation concentration ranged from 50 to 160 mg L−1 depending on the composition. The diameter of the nanoparticles (NPs) was determined by DLS and TEM. Images showed that these nano-sized objects displayed spherical and worm-like morphology with a length increasing with the hydrophilic content. Preliminary studies of drug loading and drug release with a water-insoluble model drug, namely curcumin, showed that these NPs are potential candidates for drug delivery carriers.
A New Way to Silicone-Based Peptide Polymers.
This is custom heading element
Angew. Chem.-Int. Edit. 54, 3778–3782 (2015).
Jebors, S., Ciccione, J., Al-Halifa, S., Nottelet, B., Enjalbal, C., M’Kadmi, C., Amblard, M., Mehdi, A., Martinez, J.
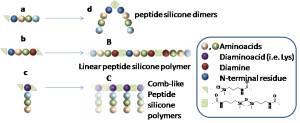
ABSTRACT
We describe a new class of silicone-peptides biopolymers obtained by a straightforward polymerization in water using tailored chlorodimethylsilyl peptides blocks as monomeric units. This general strategy is applicable to any type of peptide sequences, yielding linear or branched polymer chains composed of well defined peptide sequences.
Nomenclature and graphic representations for chemically modified polymers (IUPAC Recommendations 2014)
This is custom heading element
Pure Appl. Chem. 87, 307–319 (2015)
Jones, R. G., Kitayama, T., Wilks, E. S., Fox, R. B., Fradet, A., Hellwich, K.-H., Hess, M., Hodge, P., Horie, K., Kahovec, J., Kratochvil, P., Kubisa, P., Marechal, E., Mormann, W., Ober, C. K., Stepto, R. F. T., Vert, M. & Vohlidal, J.

Functionalized PCL/HA nanocomposites as microporous membranes for bone regeneration
This is custom heading element
Mat. Sci. Eng. C-Mater. Biol. Appl. 48, 457–468 (2015)
Basile, M. A., d’Ayala, G. G., Malinconico, M., Laurienzo, P., Coudane, J., Nottelet, B., Della Ragione, F. & Oliva, A.
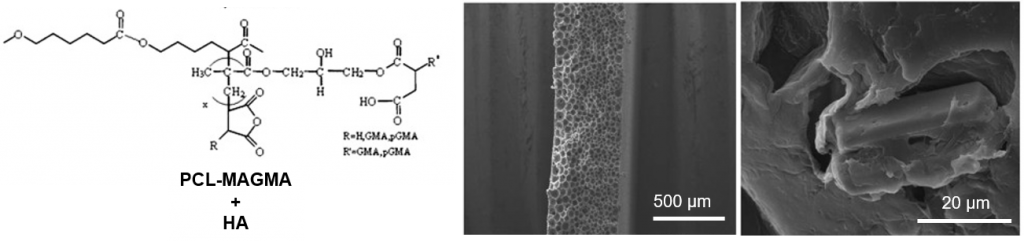
ABSTRACT
In the present work, microporous membranes based on poly(ε-caprolactone) (PCL) and PCL functionalized with amine (PCL-DMAEA) or anhydride groups (PCL-MAGMA) were realized by solvent–non solvent phase inversion and proposed for use in Guided Tissue Regeneration (GTR). Nanowhiskers of hydroxyapatite (HA) were also incorporated in the polymer matrix to realize nanocomposite membranes. Scanning Electron Microscopy (SEM) showed improved interfacial adhesion with HA for functionalized polymers, and highlighted substantial differences in the porosity. A relationship between the developed porous structure of the membrane and the chemical nature of grafted groups was proposed. Compared to virgin PCL, hydrophilicity increases for functionalized PCL, while the addition of HA influences significantly the hydrophilic characteristics only in the case of virgin polymer. A significant increase of in vitro degradation rate was found for PCL-MAGMA based membranes, and at lower extent of PCL-DMAEA membranes. The novel materials were investigated regarding their potential as support for cell growth in bone repair using multipotent mesenchymal stromal cells (MSC) as a model. MSC plated onto the various membranes were analyzed in terms of adhesion, proliferation and osteogenic capacity that resulted to be related to chemical as well as porous structure. In particular, PCL-DMAEA and the relative nanocomposite membranes are the most promising in terms of cell-biomaterial interactions.
PLA-poloxamer/poloxamine copolymers for ligament tissue engineering: sound macromolecular design for degradable scaffolds and MSC differentiation
This is custom heading element
Biomater. Sci.3, 617–626 (2015)
Leroy, A., Nottelet, B., Bony, C., Pinese, C., Charlot, B., Garric, X., Noel, D. & Coudane, J.
ABSTRACT
The treatment of anterior cruciate ligament (ACL) failures remains a current clinical challenge. The present study aims at providing suitable degradable scaffolds for ligament tissue engineering. First, we focus on the design and the evaluation of poly(lactide)/poloxamer or poly(lactide)/poloxamine multiblock copolymers selected and developed to have suitable degradation and mechanical properties to match ACL repair. In a second part it is shown that the copolymers can be processed in the form of microfibers and scaffolds consisting in a combination of twisted/braided fibers to further modulate the mechanical properties and prepare scaffold prototypes suitable for ligament application. Finally, after assessment of their cytocompatibility, the polymer scaffolds are associated to mesenchymal stem cells (MSCs). MSCs differentiation toward a ligament fibroblast phenotype is promoted by a dual stimulation including an inductive culture medium and cyclic mechanical loads. RT-qPCR analyses confirm the potential of our scaffolds and MSCs for ACL regeneration with upregulation of some differentiation markers including Scleraxis, Tenascin-C and Tenomodulin.
Turning peptides in comb silicone polymers
This is custom heading element
J. Pept. Sci. 21, 243–247 (2015)
Jebors, S., Pinese, C., Nottelet, B., Parra, K., Amblard, M., Mehdi, A., Martinez, J. & Subra, G
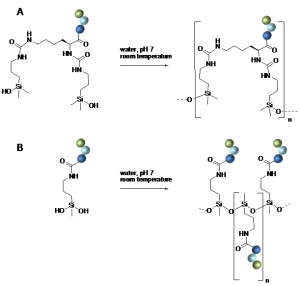
ABSTRACT
We have recently reported on a new class of silicone-peptide biopolymers obtained by polymerization of di-functionalized chlorodimethylsilyl hybrid peptides. Herein we describe a related strategy based on dichloromethylsilane-derived peptides, which yields novel polymers with a polysiloxane backbone, comparable to a silicone bearing pendent peptide chains. Interestingly, polymerization is chemoselective towards amino acids side-chains, proceeds in a single step in very mild conditions (neutral pH, water, room temperature). As potential application, a cationic sequence was polymerized and used an antibacterial coating.
MRI-visible nanoparticles from hydrophobic gadolinium poly(ε-caprolactone) conjugates
This is custom heading element
Porsio, B., Lemaire, L., El Habnouni, S., Darcos, V., Franconi, F., Garric, X., Coudane, J. & Nottelet, B
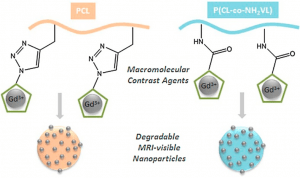
ABSTRACT
In this work we report on the synthesis of two hydrophobic and degradable gadolinium poly(ε-caprolactone) conjugates and their use for the preparation of MRI-visible nanoparticles intended for diagnosis applications. Advantage has been taken from functional poly(ε-caprolactone)s (PCL) bearing propargyl (PCL-yne) or amine groups (P(CL-co-NH2VL)) to yield conjugates by following two strategies. In a first approach, an azido-chelate of gadolinium (Gd(III)) has been conjugated by CuAAC to PCL-yne to yield a polymeric chelate containing 2.6 wt% of Gd(III). In a second approach, a dianhydride Gd(III)-ligand was reacted with P(CL-co-NH2VL) to yield, after complexation with Gd(III) salts, a polymeric chelate containing 15.4 wt% of Gd(III). The polymers biocompatibility was assessed against L929 fibroblasts. In a second part, advantage was taken from the PCLs conjugates hydrophobicity to easily prepare by nanoprecipitation nanoparticles with diameters ranging from 120 to 170 nm. The nanoparticles MRI-visibility was then evaluated and confirmed under the spin-echo and the clinically relevant gradient-echo MRI sequences.
From nanospheres to micelles: simple control of PCL-g-PEG copolymers’ amphiphilicity through thiol-yne photografting
This is custom heading element
Polym. Chem. 6, 5093–5102 (2015)
Al Samad, A., Bakkour, Y., Fanny, C., El Omar, F., Coudane, J. & Nottelet, B
ABSTRACT
A simple method for the synthesis of a family of poly(ɛ-caprolactone)-g-polyethylene glycol (PCL-g-PEG) copolymers of controlled amphiphilicity and their use to generate nanospheres or micelles are reported. PCL-g-PEG with various compositions are prepared from a single strategy relying on a combination of post-polymerization modification and subsequent thiol-yne photografting. Alkyne-functional PCL (PCL-yne) are first obtained by anionic activation and reaction with propargyl bromide to yield PCL-yne with 8% alkyne groups. PEG-thiol is then reacted on PCL-yne under UV activation to yield the targeted graft copolymer by thiol-yne photoaddition. The advantage of the approach is that control over the composition is easily achieved yielding to amphiphilic graft copolymers with ethylene glycol/ caprolactone (EG/CL) ratios ranging from 0.1 to 1.3. Starting from this single strategy, it was therefore possible to obtain nanospheres (DH~55 nm) or micelles (DH~30 nm) by copolymers self-assembly depending on the ratio EG/CL. The potential of the PCL-g-PEG micelles as drug carrier was finally evaluated with curcumin that was efficiently encapsulated, protected and released over 80 days. Interestingly it was found that drug encapsulation efficiency and drug loading were higher for PCL-g-PEG copolymers compared to block PCL-b-PEG.
Design and Development of Immunomodulatory Antigen Delivery Systems Based on Peptide/PEG-PLA Conjugate for Tuning Immunity
This is custom heading element
Biomacromolecules 16, 3666–3673 (2015)
Coumes, F., Huang, C.-Y., Huang, C.-H., Coudane, J., Domurado, D., Li, S., Darcos, V. & Huang, M.-H.
ABSTRACT
Cancer vaccines are considered to be a promising tool for cancer immunotherapy. However, a well-designed cancer vaccine should combine a tumor-associated antigen (TAA) with the most effective immunomodulatory agents and/or delivery system to provoke intense immune responses against the TAA. In the present study, we introduced a new approach by conjugating the immunomodulatory molecule LD-indolicidin to the hydrophilic chain end of the polymeric emulsifier poly(ethylene glycol)-polylactide (PEG-PLA), allowing the molecule to be located close to the surface of the resulting emulsion. A peptide/polymer conjugate, named LD-indolicidin–PEG-PLA, was synthesized by conjugation of the amine end-group of LD-indolicidin to the N-hydroxysuccinimide-activated carboxyl end-group of PEG. As an adjuvant for cancer immunotherapeutic use, TAA vaccine candidate formulated with the LD-indolicidin–PEG-PLA-stabilized squalene-in-water emulsion could effectively help to elicit a T helper (Th)1-dominant antigen-specific immune response as well as antitumor ability, using ovalbumin (OVA) protein/EG7 cells as a TAA/tumor cell model. Taken together, these results open up a new approach to the development of immunomodulatory antigen delivery systems for vaccine adjuvants and cancer immunotherapy technologies.
Thermo-responsive drug release from self-assembled micelles of brush-like PLA/PEG analogues block copolymers
This is custom heading element
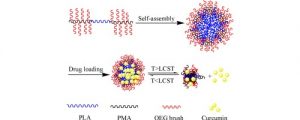
ABSTRACT
Thermo-responsive brush-like amphiphilic poly[2-(2-methoxyethoxy) ethyl methacrylate-co-oligo(ethylene glycol) methacrylate]-b-poly(l-lactide)-b-poly[2-(2-methoxyethoxy) ethyl methacrylate-co-oligo(ethylene glycol) methacrylate] [P(MEO2MA-co-OEGMA)-b-PLLA-b-P(MEO2MA-co-OEGMA)] triblock copolymers were synthesized by atom transfer radical polymerization of MEO2MA and OEGMA co-monomers using a α,ω-Bromopropionyl poly(l-lactide) (Br-PLLA-Br) macroinitiator. The resulting copolymers with MEO2MA/OEGMA molar ratio ranging from 79/21 to 42/58 were characterized by 1H nuclear magnetic resonance and size exclusion chromatography. Thermo-responsive micelles were obtained by self-assembly of copolymers in aqueous medium. The micelles are spherical in shape with sizes varying from 20.7 to 102.5 nm. A hydrophobic anticancer drug, curcumin, was encapsulated in micelles by using membrane hydration method. The properties of drug loaded micelles were determined by dynamic light scattering, transmission electron microscopy and lower critical solution temperature (LCST) measurements. The micelles size decreases from 102.5 nm for blank micelles to 37.6 nm with 10.8% drug loading, suggesting that the drug plays an important role in the micellization procedure. The LCST decreases from 45.1 °C for blank micelles to 40.6 and 38.3 °C with 5.9 and 10.8% drug loading, respectively. In vitro drug release was performed in pH 7.4 PBS at different temperatures. Data show that the release rate was significantly enhanced above the LCST comparing with that below the LCST. The amount of released drug at 41 °C was ca. 20% higher than that at 37 °C. Burst-like release was depressed due to enhanced interaction between drug with hydrophobic PLA and PMA chains.
Biocompatibility of thermo-responsive PNIPAAm-PLLA-PNIPAAm triblock copolymer as potential drug carrier.
This is custom heading element

ABSTRACT
This work aims to evaluate the cytocompatibility and hemocompatibility of thermo‐responsive polymers as potential drug carrier. Thermo‐responsive poly(N‐isopropyl acrylamide) (PNIPAAm) and poly(N‐isopropyl acrylamide)‐poly(l‐lactide)‐poly(N‐isopropyl acrylamide) (PNIPAAm‐PLLA‐PNIPAAm) triblock copolymer were synthesized by atom transfer radical polymerization using ethyl α‐bromoisobutyrate or Br‐PLLA‐Br as initiator under mild conditions. The self‐assembly and thermo‐responsive properties of the copolymer in aqueous medium were investigated by critical micelle concentration, dynamic light scattering, transmission electron microscopy, and lower critical solution temperature measurements. The critical micelle concentration was 0.014 mg ml−1. Dynamic light scattering and transmission electron microscopy results show that the micelles are spherical in shape with sizes between 20 and 40 nm. The lower critical solution temperature of PNIPAAm and PNIPAAm‐PLLA‐PNIPAAm is 34.8°C and 32.8°C, respectively. 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide assay was carried out to evaluate the cytotoxicity of polymers, and the hemocompatibility was assessed from hemolysis ratio and plasma recalcification time measurements. The results show that PNIPAAm‐PLLA‐PNIPAAm presents outstanding biocompatibility and could be promising for applications in targeted drug delivery.
Bioinspired Titanium Drug Eluting Platforms Based on a Poly-beta-cyclodextrin-Chitosan Layer-by-Layer Self-Assembly Targeting Infections
This is custom heading element
ACS Appl. Mater. Interfaces 7, 12882–12893 (2015)
Perez-Anes, A., Gargouri, M., Laure, W., Van Den Berghe, H., Courcot, E., Sobocinski, J., Tabary, N., Chai, F., Blach, J.-F., Addad, A., Woisel, P., Douroumis, D., Martel, B., Blanchemain, N. & Lyskawa, J.
ABSTRACT
In the field of implantable titanium-based biomaterials, infections and inflammations are the most common forms of postoperative complications. The controlled local delivery of therapeutics from implants through polyelectrolyte multilayers (PEMs) has recently emerged as a versatile technique that has shown great promise in the transformation of a classical medical implant into a drug delivery system. Herein, we report the design and the elaboration of new biodegradable multidrug-eluting titanium platforms based on a polyelectrolyte multilayer bioactive coating that target infections. These systems were built up in mild conditions according to the layer-by-layer (L-b-L) assembly and incorporate two biocompatible polysaccharides held together through electrostatic interactions. A synthetic, negatively charged β-cyclodextrin-based polymer (PCD), well-known for forming stable and reversible complexes with hydrophobic therapeutic agents, was exploited as a multidrug reservoir, and chitosan (CHT), a naturally occurring, positively charged polyelectrolyte, was used as a barrier for controlling the drug delivery rate. These polyelectrolyte multilayer films were strongly attached to the titanium surface through a bioinspired polydopamine (PDA) film acting as an adhesive first layer and promoting the robust anchorage of PEMs onto the biomaterials. Prior to the multilayer film deposition, the interactions between both oppositely charged polyelectrolytes, as well the multilayer growth, were monitored by employing surface plasmon resonance (SPR). Several PEMs integrating 5, 10, and 15 bilayers were engineered using the dip coating strategy, and the polyelectrolyte surface densities were estimated by colorimetric titrations and gravimetric analyses. The morphologies of these multilayer systems, as well as their naturally occurring degradation in a physiological medium, were investigated by scanning electron microscopy (SEM), and their thicknesses were measured by means of profilometry and ellipsometry studies. Finally, the ability of the coated titanium multilayer devices to act as a drug-eluting system and to treat infections was validated with gentamicin, a relevant water-soluble antibiotic commonly used in medicine due to its broad bactericidal spectrum.
Tolerance and Long-Term MRI Imaging of Gadolinium-Modified Meshes Used in Soft Organ Repair
This is custom heading element
Letouzey, V., Huberlant, S., Cornille, A., Blanquer, S., Guillaume, O., Lemaire, L., Garric, X. & de Tayrac, R.
ABSTRACT
Background
Synthetic meshes are frequently used to reinforce soft tissues. The aim of this translational study is to evaluate tolerance and long-term MRI visibility of two recently developed Gadolinium-modified meshes in a rat animal model.
Materials and Methods
Gadolinium-poly-ε-caprolactone (Gd-PCL) and Gadolinium-polymethylacrylate (Gd-PMA) modified meshes were implanted in Wistar rats and their tolerance was assessed daily. Inflammation and biocompatibility of the implants were assessed by histology and immunohistochemistry after 30 days post implantation. Implants were visualised by 7T and 3T MRI at day 30 and at day 90. Diffusion of Gadolinium in the tissues of the implanted animals was assessed by Inductively Coupled Plasma Mass Spectrometry.
Results
Overall Gd-PMA coated implants were better tolerated as compared to those coated with Gd-PCL. In fact, Gd-PMA implants were characterised by a high ratio collagen I/III and good vascularisation of the integration tissues. High resolution images of the coated mesh were obtained in vivo with experimental 7T as well as 3T clinical MRI. Mass spectrometry analyses showed that levels of Gadolinium in animals implanted with coated mesh were similar to those of the control group.
Conclusions
Meshes coated with Gd-PMA are better tolerated as compared to those coated with Gd-PCL as no signs of erosion or significant inflammation were detected at 30 days post implantation. Also, Gd-PMA coated meshes were clearly visualised with both 7T and 3T MRI devices. This new technique of mesh optimisation may represent a valuable tool in soft tissue repair and management.
Radiopaque poly(epsilon-caprolactone) as additive for X-ray imaging of temporary implantable medical devices
This is custom heading element
RSC Adv. 5, 84125–84133 (2015)
Samuel, R., Girard, E., Chagnon, G., Dejean, S., Favier, D., Coudane, J. & Nottelet, B
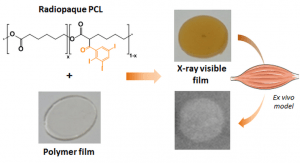
ABSTRACT
In this work we report on the synthesis of two hydrophobic and degradable gadolinium poly(ε-caprolactone) conjugates and their use for the preparation of MRI-visible nanoparticles intended for diagnosis applications. Advantage has been taken from functional poly(ε-caprolactone)s (PCL) bearing propargyl (PCL-yne) or amine groups (P(CL-co-NH2VL)) to yield conjugates by following two strategies. In a first approach, an azido-chelate of gadolinium (Gd(III)) has been conjugated by CuAAC to PCL-yne to yield a polymeric chelate containing 2.6 wt% of Gd(III). In a second approach, a dianhydride Gd(III)-ligand was reacted with P(CL-co-NH2VL) to yield, after complexation with Gd(III) salts, a polymeric chelate containing 15.4 wt% of Gd(III). The polymers biocompatibility was assessed against L929 fibroblasts. In a second part, advantage was taken from the PCLs conjugates hydrophobicity to easily prepare by nanoprecipitation nanoparticles with diameters ranging from 120 to 170 nm. The nanoparticles MRI-visibility was then evaluated and confirmed under the spin-echo and the clinically relevant gradient-echo MRI sequences.
Aliphatic polyesters for medical imaging and theranostic applications
This is custom heading element
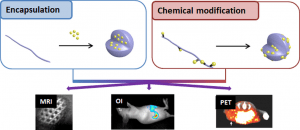
ABSTRACT
Medical imaging is a cornerstone of modern medicine. In that context the development of innovative imaging systems combining biomaterials and contrast agents (CAs)/imaging probes (IPs) for improved diagnostic and theranostic applications focuses intense research efforts. In particular, the classical aliphatic (co)polyesters poly(lactide) (PLA), poly(lactide-co-glycolide) (PLGA) and poly(e-caprolactone) (PCL), attract much attention due to their long track record in the medical field. This review aims therefore at providing a state-of-the-art of polyester-based imaging systems. In a first section a rapid description of the various imaging modalities, including magnetic resonance imaging (MRI), optical imaging, computed tomography (CT), ultrasound (US) and radionuclide imaging (SPECT, PET) will be given. Then, the two main strategies used to combine the CAs/IPs and the polyesters will be discussed. In more details we will first present the strategies relying on CAs/IPs encapsulation in nanoparticles, micelles, dendrimers or capsules. We will then present chemical modifications of polyesters backbones and/or polyester surfaces to yield macromolecular imaging agents. Finally, opportunities offered by these innovative systems will be illustrated with some recent examples in the fields of cell labeling, diagnostic or theranostic applications and medical devices.
Development of pharmaceutical clear gel based on Peceol (R), lecithin, ethanol and water: Physicochemical characterization and stability study
This is custom heading element
J. Colloid Interface Sci. 457, 152–161, (2015)
Mouri, A., Diat, O., Lerner, D. A., El Ghzaoui, A., Ly I., Dorandeu C., Maurel, J.-C., Devoisselle, J.-M., Dorandeu, C., Legrand, P.
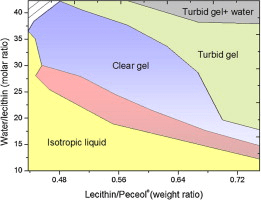
ABSTRACT
The phase behavior of the four-components Peceol®/lecithin/ethanol/water system has been studied in a part of the phase diagram poor in water and varying the lecithin/Peceol® ratio. Using several complementary techniques such as Karl Fischer titration, rheology, polarized microscopy and SAXS measurements several nanostructures of the complex systems were identified. W/O microemulsion (L2) as well as an inverted hexagonal (H2) liquid-crystal phase were studied. The analysis of the different phase transitions allows us to understand the effect of lecithin on the water solubilization efficiency of this clear gel and to show its pharmaceutical interest among lecithin organogels.
Modulating Viscoelastic Properties of Physically Cross linked Self-Assembled Gelatin Hydrogels through Optimized Solvent Conditions
This is custom heading element
J. Dispers. Sci. Technol. 36, 1349-1356, (2015)
Kadam, K., Pochat-Bohatier C., Sanchez J., El Ghzaoui A.

ABSTRACT
In this work, an experimental study was conducted on two different types of gelatin from mammalian sources, type A, derived from acid-cured tissue of porcine skin and type B, from lime-cured tissue of bovine skin. The effect of temperature, pH, and ionic strength on viscoelastic properties of gelatin hydrogels was carried out in order to determine the conformational characteristics and phase transition (sol-gel transition temperatures, Tm – melting temperature and T-g- gelling temperature) behavior. The detailed investigation on gelation behavior of gelatin hydrogels was performed with rheological measurements in the temperature range of 5-35 degrees C as a function of pH with and without added salt (NaCl) and gelation transition temperatures evaluated for all these conditions. Both types of gelatins show polyelectrolyte effect with added NaCl (0.001-0.1 M) for the studied pH conditions (pH 1 or pH 7). The maximum in the gelation properties was observed at lower NaCl concentrations (0.001 M for type-A and 0.01 M for type-B). The significant improvement in gelation behavior with optimum pH or amount of added NaCl imparts longer stability to gelatin hydrogels, which were reflected in degradation study. This work establishes a relationship between gelatin properties and the values of temperatures and solvent conditions (pH and NaCl concentrations), thus providing insight on how to control the stability and melting of gelatin gels in view of their potential application in the biomedical and food industries.
Thermo-responsive release of curcumin from micelles prepared by self-assembly of amphiphilic P(NIPAAm-co-DMAAm)-b-PLLA-b-P (NIPAAm-co-DMAAm) triblock copolymers
This is custom heading element
Int. J. Pharm. 476, 31–40 (2014).
Hu, Y., Darcos, V., Monge, S., Li, S., Zhou, Y. & Su, F.
Water solubilization capacity of pharmaceutical microemulsions based on Peceol (R), lecithin and ethanol.
This is custom heading element
Int. J. Pharm. 475, 324–334 (2014).
Mouri, A., Diat, O., Lerner, D. A., El Ghzaoui, A., Ajovalasit, A., Dorandeu, C., Maurel, J.-C., Devoisselle, J.-M. & Legrand, P.
When Functionalization of PLA Surfaces Meets Thiol–Yne Photochemistry: Case Study with Antibacterial Polyaspartamide Derivatives.
This is custom heading element
Biomacromolecules 15, 4351–4362 (2014).
Sardo, C., Nottelet, B., Triolo, D., Giammona, G., Garric, X., Lavigne, J.-P., Cavallaro, G. & Coudane, J.
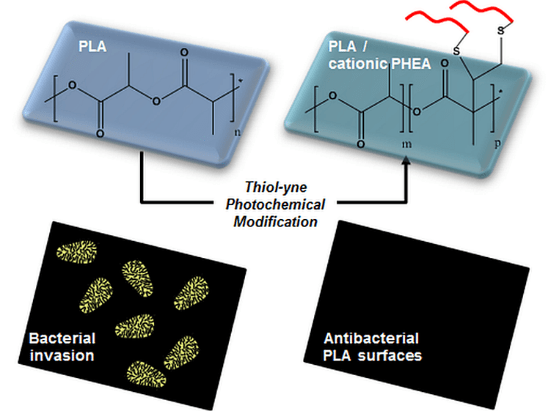
ABSTRACT
The objective of this work was to develop and study new biodegradable thermoplastics with improved mechanical properties for potential use as temporary implantable biomaterials. Linear Poloxamer and star-shaped Poloxamine have been used as macroinitiators for the Ring-Opening Polymerization (ROP) of lactide to yield high molecular weight PLA-based thermoplastic block copolymers. The influence of the nature of the macroinitiator, PLA crystallinity and initial molecular weight on the copolymers properties was investigated by performing a 7-week degradation test in PBS. The evaluation of water uptakes and molecular weights during the degradation pointed out an early hydrolytic degradation of the 100 kg∙mol-1 copolymers compared to the 200 kg/mol ones (molecular weight decrease of ca. 40 % and 20 %, respectively). A dramatic loss of tensile mechanical properties was also observed for the 100 kg/mol copolymers whereas the 200 kg∙mol-1 copolymers showed stable or even slightly improved properties with Young’s moduli around 500 MPa and yield strains around 3 to 4%. Finally, the cytocompatibility of the more stable 200 kg∙mol-1 copolymers was confirmed by murine mesenchymal stem cells (MSCs) culture.
Abbreviations of polymer names and guidelines for abbreviating polymer names (IUPAC Recommendations 2014).
This is custom heading element
Pure Appl. Chem. 86, 1003–1015 (2014).
He, J., Chen, J., Hellwich, K.-H., Hess, M., Horie, K., Jones, R. G., Kahovec, J., Kitayama, T., Kratochvil, P., Meille, S. V., Mita, I., dos Santos, C., Vert, M. & Vohlidal, J.
Experimental and Theoretical Studies of Polyanion-Polycation Complexation in Salted Media in the Context of Nonviral Gene Transfection
This is custom heading element
Macromolecules 47, 3574–3581 (2014).
Boustta, M., Leclercq, L., Vert, M. & Vasilevskaya, V. V.
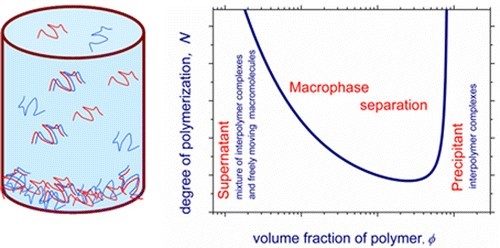
ABSTRACT
In the field of biological applications, polyelectrolyte complexes are proposed to encapsulate bioactive compounds, to deliver drugs, and also to transfect genes into cells under the name of polyplexes. Complex formation is obtained by addition of a polycation solution into a polyanion solution or vice-versa. This work proposes a theoretical approach to describe complex formation in the case of non-stoichiometric mixtures of oppositely charged macroions having different degrees of ionization and different degrees of polymerization under different salt conditions. In a second part, comparison was made with experimental data collected when a weak polybase, namely poly(L-lysine) under its bromide form was added stepwise to solutions of various polyanions under their sodium salt form, namely poly(L-lysine citramide imide), poly(L-lysine citramide), and poly(b-malic acid), the latter lacking hydroxyl groups attached to the main chain. The stability of stroichiometric complexes made of poly(L-lysine) and poly(L-lysine citramide) having different molecular masses is discussed
Biodegradable networks for soft tissue engineering by thiol–yne photo cross-linking of multifunctional polyesters.
This is custom heading element
RSC Adv. 4, 32017–32023 (2014).
Leroy, A., Al Samad, A., Garric, X., Hunger, S., Noël, D., Coudane, J. & Nottelet, B.
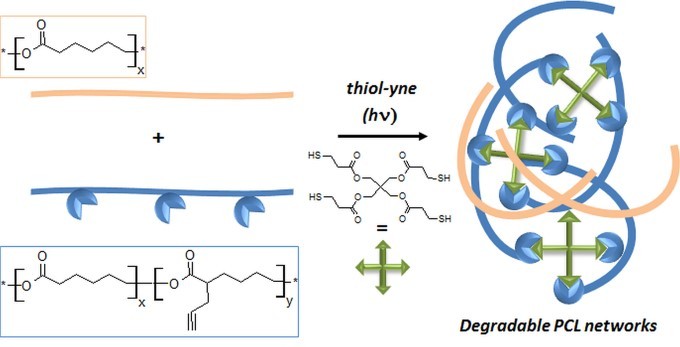
ABSTRACT
Novel biodegradable networks have been prepared by combination of post-polymerization modification of poly(e-caprolactone) and thiol-yne photo cross-linking. In a first step, a novel multifunctional poly(e-caprolactone) bearing propargyl groups (PCL-yne, 8 mol% substitution degree) was obtained in a rapid one-pot reaction and at a multigram scale. In a second step, PCL-yne and mixtures of PCL and PCL-yne were cross-linked via photoradical thiol-yne reaction in the presence of pentaerythritol tetrakis(3-mercaptopropionate) and various photoinitiators. The thermal properties and stabilities of the obtained networks have been evaluated before assessment of their mechanical properties. Finally, biocompatibility studies are reported with evaluation of the proliferation of L929 fibroblasts on the materials.
Regioselective Halogenation of Poly(lactide) by Free-Radical Process.
This is custom heading element
Macromol. React. Eng. 8, 141–148 (2014).
Beuille, E., Darcos, V., Coudane, J., Lacroix-Desmazes, P. & Nottelet, B.
ABSTRACT
Modification of the biodegradable and renewable poly(lactide) (PLA) to provide functional aliphatic polyesters is highly desirable to meet applications requirements. In this context, the objective of this work was to evaluate the possibility to use free-radical substitution as a simple and non-degrading method to yield the desired functional PLA. Halogenation of PLA was carried out in solution and various conditions of reaction have been evaluated. Halogenated PLAs were characterized by 1H NMR spectroscopy and size exclusion chromatography (SEC) to evaluate the substitution ratios and to check the non-degrading character of the radical modification. Furthermore, reactions on model compounds as well as MALDI-TOF analyses have been performed to elucidate the involved mechanism. The combined results proved the free-radical halogenation of PLA in solution to be regioselective with only the w-halogenated PLA being obtained without main-chain substitution.
Versatile UCST-based thermoresponsive hydrogels for loco- regional sustained drug delivery.
This is custom heading element
Control. Release 174, 1–6 (2014).
Boustta, M., Colombo, P.-E., Lenglet, S., Poujol, S. & Vert, M.
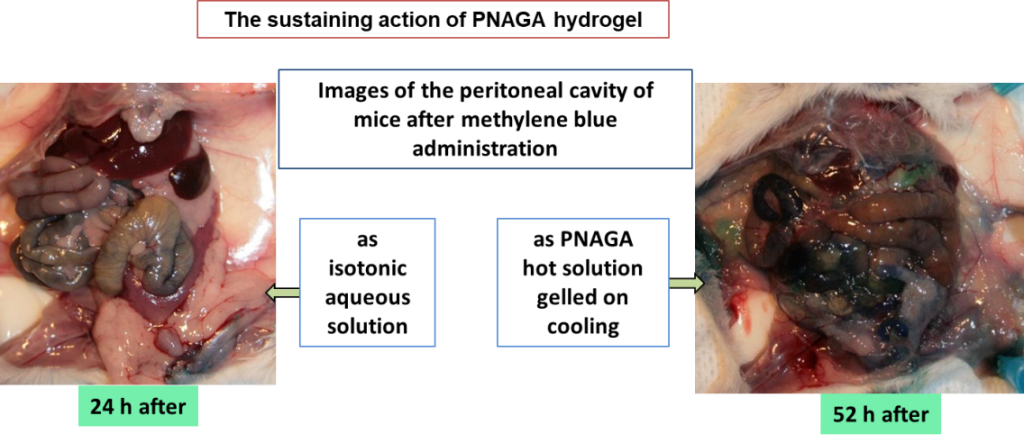
ABSTRACT
Modification of the biodegradable and renewable poly(lactide) (PLA) to provide functional aliphatic polyesters is highly desirable to meet applications requirements. In this context, the objective of this work was to evaluate the possibility to use free-radical substitution as a simple and non-degrading method to yield the desired functional PLA. Halogenation of PLA was carried out in solution and various conditions of reaction have been evaluated. Halogenated PLAs were characterized by 1H NMR spectroscopy and size exclusion chromatography (SEC) to evaluate the substitution ratios and to check the non-degrading character of the radical modification. Furthermore, reactions on model compounds as well as MALDI-TOF analyses have been performed to elucidate the involved mechanism. The combined results proved the free-radical halogenation of PLA in solution to be regioselective with only the w-halogenated PLA being obtained without main-chain substitution.
MRI-Visible Poly(ε-caprolactone) with Controlled Contrast Agent Ratios for Enhanced Visualization in Temporary Imaging Applications.
This is custom heading element
Biomacromolecules 14, 3626–3634 (2013).
El Habnouni, S., Nottelet, B., Darcos, V., Porsio, B., Lemaire, L., Franconi, F., Garric, X. & Coudane, J.
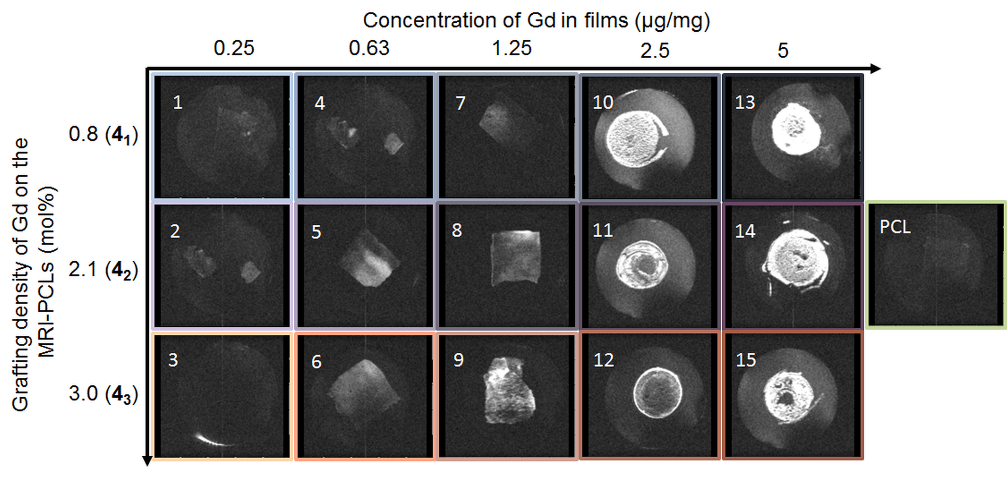
ABSTRACT
Hydrophobic macromolecular contrast agents (MMCAs) are highly desirable to provide safe and efficient magnetic resonance (MR) visibility to implantable medical devices. In this study, we report on the synthesis and evaluation of novel biodegradable poly(-caprolactone)-based MMCAs. Poly(α-propargyl-ε-caprolactone-co-ε-caprolactone)s containing 2, 5 and 10 mol% of propargyl groups have been prepared by ring opening copolymerization of -caprolactone and the corresponding propargylated lactone. In parallel, a di-azido derivative of the clinically used diethylenetriaminepentaacetic acid (DTPA) / Gd3+ complex has been synthesized. Finally, MRI-visible poly(-caprolactone)s (PCLs) were obtained by the efficient click ligation of these compounds via a CuI-catalyzed [3+2] cycloaddition. ICP-MS analyses confirmed the efficient coupling of the complex on the PCL backbone with the MRI-visible PCLs containing 1.0, 2.6 and 3.6 wt% of Gd3+. The influence of the Gd3+ grafting density on the T1 relaxation times and on the MRI-visibility of the novel biodegradable MMCAs was evaluated. Finally, their stability and cytocompatibility were assessed with regard to their potential as innovative MRI-visible biomaterials for biomedical applications.
Toward potent antibiofilm degradable medical devices: A generic method for the antibacterial surface modification of polylactide.
This is custom heading element
Acta Biomaterialia 9, 7709–7718 (2013)
El Habnouni, S., Lavigne, J.-P., Darcos, V., Porsio, B., Garric, X., Coudane, J. & Nottelet, B.
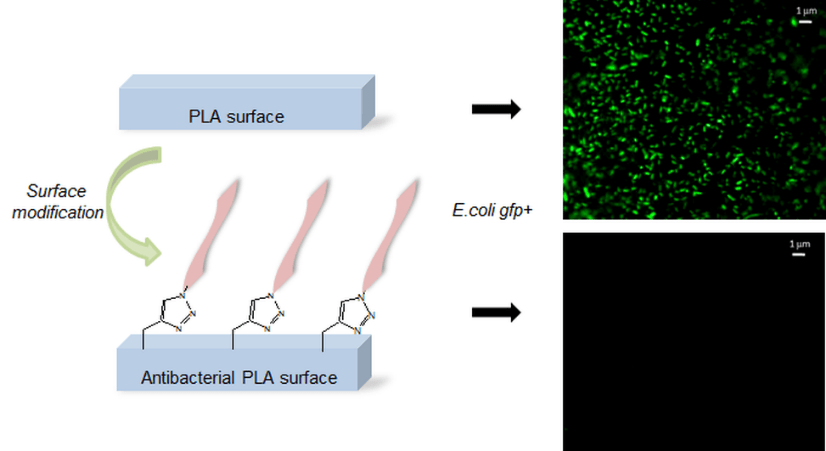
ABSTRACT
The effects of biomaterials on their environment must be carefully modulated in most biomedical applications. Among other approaches, this modulation can be obtained through the modification of the biomaterial surface. This paper proposes a simple and versatile strategy to produce non-leaching
antibacterial polylactide (PLA) surfaces without any degradation of the polyester chains. The method is based on a one-pot procedure that provides a ‘‘clickable’’ PLA surface via anionic activation which is then functionalized with an antibacterial quaternized poly(2-(dimethylamino)ethyl methacrylate) (QPDMAEMA) by covalent immobilization on the surface. The anti-adherence and antibiofilm activities of modified PLA surfaces are assessed for different QPDMAEMA molecular weights and different quaternization agents. Antibacterial PLA surfaces are shown to be very active against Gram-negative and Gram-positive strains, with adherence reduction factors superior to 99.999% and a marked reduction in biofilm on the most potent surfaces. In addition to this substantial antibacterial activity, the proposed PLA surfaces are also cytocompatible, as demonstrated through the proliferation of L929 fibroblasts.
Complex impedance spectroscopy to investigate degradable chondroitin-poly(amino-serinate) complexes
This is custom heading element
Polym. Degrad. Stabil. 98, 2161–2167 (2013).
Balme, S., Rixte, J., Boustta, M., Vert, M. & Henn, F.
ABSTRACT
Sodium chondroitin-4-sulfate and poly(amino-serinate) bromide can interact to form a degradable polyelectrolyte complex. The structure of each polymer and of their complex before and after degradation is investigated by complex impedance spectroscopy. Poly(amino-serinate) bromide and sodium chondroitin-4-sulfate exhibit dc conductivity and dielectric relaxation phenomena in the 102e106 Hz range. On the contrary, no dc conductivity and dielectric relaxation are observed in the polyelectrolyte complex before degradation. After degradation, the released chondroitin-4-sulfate is re-complexed with additional poly(amino-serinate) bromide. Contrary to the original parent complex the restored complex exhibits both dc conductivity and dielectric relaxation phenomena. This difference is assigned to structural defects due to the presence of residual poly(amino-serinate) oligomers which compete with the newly added poly(amino-serinate) to complex the released chondroitin-4-sulfate. This outcome is interpreted assuming the displacement of the low molecular by higher molecular weight chains in contrast to the behavior usually reported for this type of polymeric systems.
New PLGA–P188–PLGA matrix enhances TGF-β3 release from pharmacologically active microcarriers and promotes chondrogenesis of mesenchymal stem cells.
This is custom heading element
Journal of Controlled Release 170, 99–110 (2013).
Morille, M., Van-Thanh, T., Garric, X., Cayon, J., Coudane, J., Noël, D., Venier-Julienne, M.-C. & Montero-Menei, C. N.
Investigation on the properties of linear PLA-poloxamer and star PLA-poloxamine copolymers for temporary biomedical applications.
This is custom heading element
Materials Science and Engineering: C 33, 4133–4139 (2013).
Leroy, A., Pinese, C., Bony, C., Garric, X., Noël, D., Nottelet, B. & Coudane, J.
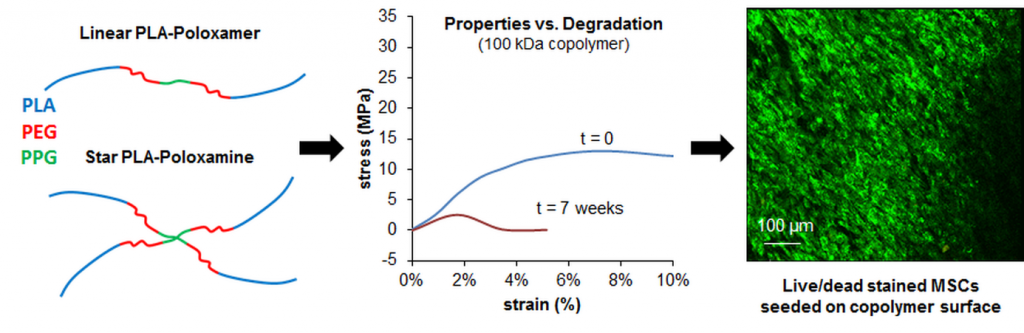
ABSTRACT
The objective of this work was to develop and study new biodegradable thermoplastics with improved mechanical properties for potential use as temporary implantable biomaterials. Linear Poloxamer and star-shaped Poloxamine have been used as macroinitiators for the Ring-Opening Polymerization (ROP) of lactide to yield high molecular weight PLA-based thermoplastic block copolymers. The influence of the nature of the macroinitiator, PLA crystallinity and initial molecular weight on the copolymers properties was investigated by performing a 7-week degradation test in PBS. The evaluation of water uptakes and molecular weights during the degradation pointed out an early hydrolytic degradation of the 100 kg∙mol-1 copolymers compared to the 200 kg/mol ones (molecular weight decrease of ca. 40 % and 20 %, respectively). A dramatic loss of tensile mechanical properties was also observed for the 100 kg/mol copolymers whereas the 200 kg∙mol-1 copolymers showed stable or even slightly improved properties with Young’s moduli around 500 MPa and yield strains around 3 to 4%. Finally, the cytocompatibility of the more stable 200 kg∙mol-1 copolymers was confirmed by murine mesenchymal stem cells (MSCs) culture.
Synthesis of peptide-grafted comb polypeptides via polymerisation of NCA-peptides.
This is custom heading element
Chem. Commun. 49, 409–411 (2013).
Enomoto, H., Nottelet, B., Al Halifa, S., Enjalbal, C., Dupre, M., Tailhades, J., Coudane, J., Subra, G., Martinez, J. & Amblard, M
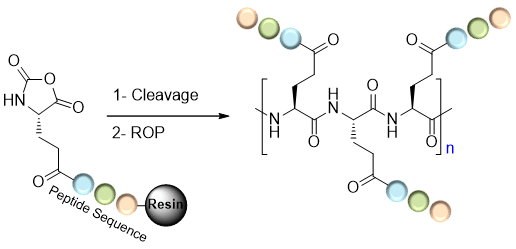
ABSTRACT
The objective of this work was to develop and study new biodegradable thermoplastics with improved mechanical properties for potential use as temporary implantable biomaterials. Linear Poloxamer and star-shaped Poloxamine have been used as macroinitiators for the Ring-Opening Polymerization (ROP) of lactide to yield high molecular weight PLA-based thermoplastic block copolymers. The influence of the nature of the macroinitiator, PLA crystallinity and initial molecular weight on the copolymers properties was investigated by performing a 7-week degradation test in PBS. The evaluation of water uptakes and molecular weights during the degradation pointed out an early hydrolytic degradation of the 100 kg∙mol-1 copolymers compared to the 200 kg/mol ones (molecular weight decrease of ca. 40 % and 20 %, respectively). A dramatic loss of tensile mechanical properties was also observed for the 100 kg/mol copolymers whereas the 200 kg∙mol-1 copolymers showed stable or even slightly improved properties with Young’s moduli around 500 MPa and yield strains around 3 to 4%. Finally, the cytocompatibility of the more stable 200 kg∙mol-1 copolymers was confirmed by murine mesenchymal stem cells (MSCs) culture.