Polymer and diagnostics
Polymers for medical imaging
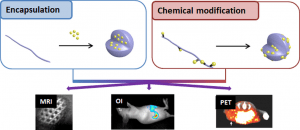
MRI visible polymers for coatings and theranostic applications
About the project:
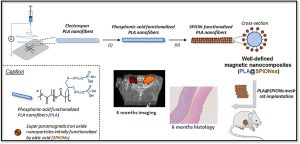
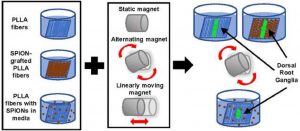
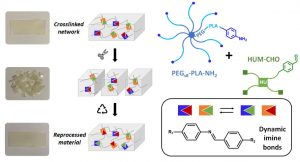
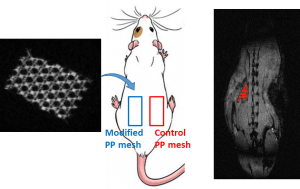
This project aims at offering chemical strategies that can help making the polymeric implants visible by the clinically relevant MRI procedures.For that, we focus on novel macromolecular MRI contrast agents that can be used as coatings, or as micro- and nanoparticles, and we focus on surface modification to make existing polymers MRI-visible.
Contact:
Students:
Anita Shulz
Mira Younis
Sarah El Habnouni
Collaborations:
Dr. Lemaire (MINT, Inserm 1066 – CNRS 6021), Dr. Franconi (platform PRISM), Prof. Letouzey (CHU Nîmes)
Funding:
Campus France, Post-doctoral program UM, MENRT grant (ED 459), Chercheur d’Avenir 2013
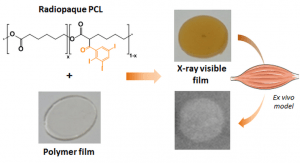
X-ray visible polymers for implantable medical devices
About the project:
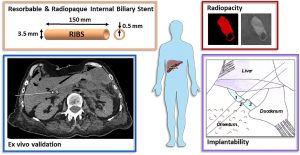
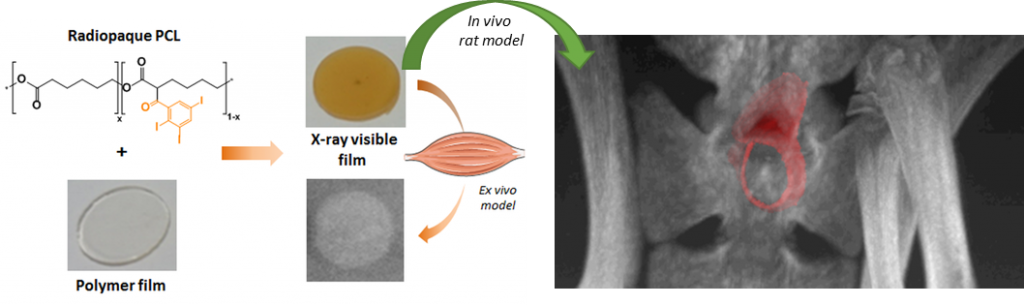
This project aims at offering chemical strategies that can help making the polymeric implants visible by the clinically relevant scanner procedures. For that, we focus on novel macromolecular MRI contrast agents that can be used as coatings, or as X-ray radiopaque agent to be formulated in medical devices.
Contact:
Students:
Edouard Girard
Collaborations:
Dr. Rhiele (University of Glasgow)), Dr. Chagnon & Prof .Favier (TIMC-IMAG, UMR 5525)
Funding:
–
Polymers for in vitro diagnostics
Project Biosensors
About the project:
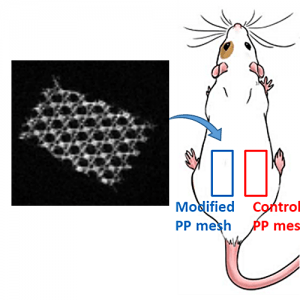
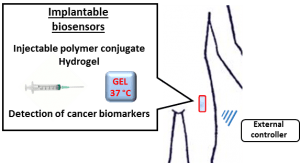
Implantable biosensors for cancer biomarkers detection. The project is focused on the development of implantable biosensors based on polymer-peptide conjugate for cancer biomarkers detection.
Contact:
Students:
Collaborations:
Dr May Morris (Institut des Biomolécules Max Mousseron), Pr. Pascal Pujol (CHU Montpellier)
Funding:
Key Initiative MUSE « Biomarkers & Therapy », Université de Montpellier