Benjamin Nottelet

Benjamin Nottelet
Professor, Faculty of pharmacy, University of Montpellier
Benjamin initially graduated as a chemical engineer from the Ecole Nationale Supérieure de Chimie of Montpellier (ENSCM), France before completing an industrial PhD on degradable polymers from the University of Montpellier (UM) in 2005 in contract with RHODIA in the group of Prof. Vert. He then worked in the Macromolecular Engineering and Architectures group of ENSCM in the group of Prof. Boutevin before joining the Department of Pharmaceutics and Biopharmaceutics of Prof. Gurny at the University of Geneva (UNIGE) to develop scaffolds for tissue engineering and drug delivery systems. In 2008, he became Associate Professor in the Faculty of Pharmacy at UM and joined the Department of Artificial Biopolymers of IBMM of Prof. Coudane, where he was appointed Full Professor in 2018. His research activities focus on the synthesis and modification of degradable polymers for advanced biomedical applications and include hybrid biomaterials, bioactive surfaces, and multifunctional polymers.
Part of his recent work focuses on the synthesis and design of (1) innovative multifunctional degradable polymers for use in the field of drug delivery with smart and stimuli-responsive systems or (2) macromolecular contrast agents in the field of diagnostic allowing for MRI or X-ray imaging in of medical devices, or of theranostic approaches.
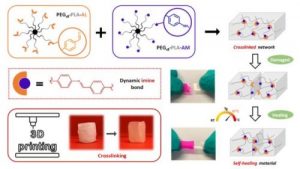
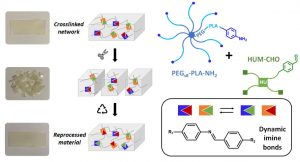
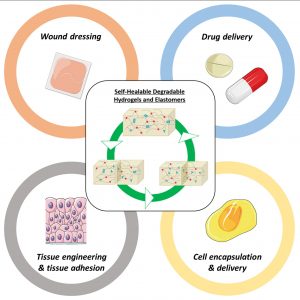
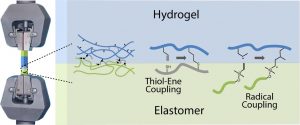
Another part of his research focuses on (3) hybrid biomaterials including peptide-based polymers or nanocomposites, and degradable elastomers for tissue engineering applications, as well as the development of (4) surface modification strategies to yield active surfaces in the frame of antibacterial and of imaging applications.
Benjamin is member of the editorial board of Multifunctional Materials and is co-author of over 70 papers and 3 patents.
Contact:
Benjamin.nottelet(a)umontpellier.fr
+33(0)4-11-75-96-97
Orcid n°: 0000-0002-8577-9273
5 recent papers :
A. El Jundi, M. Morille, N. Bettache, A. Bethry, J. Berthelot, J. Salvador, S. Hunger, Y. Bakkour, E. Belamie, B. Nottelet. Degradable double hydrophilic block copolymers and tripartite polyionic complex micelles thereof for small interfering ribonucleic acids (siRNA) delivery J. Colloid. Interface Sci. 2020,580, 449.
Girard E., Chagnon G., Broisat A., Dejean S., Soubies A., Gil H., Sharkawi T., Boucher F. Roth G.S., Trilling B., Nottelet B.From in vitro evaluation to human post-mortem pre-validation of a radiopaque and resorbable internal biliary stent for liver transplantation applications. Acta Biomater. 2020, 106, 66.
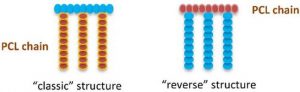
Hussein Awada, Assala Al Samad, Danielle Laurencin,* Ryan Gilbert, Xavier Dumail, Ayman El Jundi, Audrey Bethry, Rebecca Pomrenke, Christopher Johnson, Laurent Lemaire, Florence Franconi, Gautier Félix, Joulia Larionova, Yannick Guari, Benjamin Nottelet*, Controlled Anchoring of Iron Oxide Nanoparticles on Polymeric Nanofibers: Easy Access to Core@Shell Organic−Inorganic Nanocomposites for Magneto-Scaffolds ACS Appl. Mater. Interfaces 2019, 11, 9519.
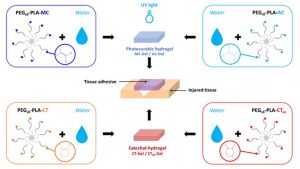
Anita Schulz, Laurent Lemaire, Audrey Bethry, Lucie Allègre, Maïda Cardoso, Florence Bernex, Florence Franconi, Christophe Goze-Bac, Hubert Taillades, Xavier Garric, Benjamin Nottelet. UV-triggered photoinsertion of contrast agent onto polymer surface for in vivo MRI-visible medical devices. Multifunctional Materials 2019, 2, 0240012019.
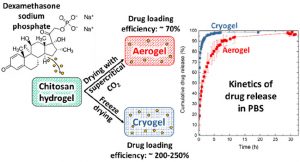
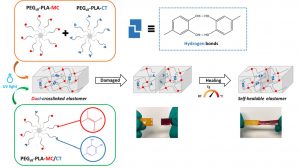
Louis Gangolphe, Stéphane Déjean, Audrey Bethry, Sylvie Hunger, Coline Pinese, Xavier Garric, Frédéric Bossard, Benjamin Nottelet. Degradable multi(aryl-azide) star copolymer as universal photo-crosslinker for elastomeric scaffolds Mat. Today Chem. 2019, 12, 209.